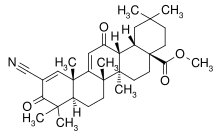
BARDOXOLONE METHYL
Methyl 2-cyano-3,12-dioxooleana-1,9(11)dien-28-oate
methyl 2-cyano-3, 12-dioxooleana-1,9(11)-dien-28-oate
2-Cyano-3,12-dioxoolean-1,9(11)-dien-28-oic acid methyl ester
(6aR,6bS,8aR,12aS,14aR,14bS)-11-Cyano-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,3,4,5,6,6a,6b,7,8,8a,9,10,12a,14,14a,14b-hexadecahydropicene-4a(2H)-carboxylic acid methyl ester
BARD
CDDO-Me
Methyl-CDDO
NSC-713200
RTA-402
TP-155C
218600-53-4 CAS
218600-44-3 (free acid)
Bardoxolone methyl (also known as “RTA 402” and “CDDO-methyl ester”) is an orally-available first-in-class synthetic triterpenoid. It is an inducer of the Nrf2 pathway, which can suppress oxidative stress and inflammation, and is undergoing clinical development for the treatment of advanced chronic kidney disease (CKD) in type 2 diabetes mellitus patients.
Bardoxolone methyl was previously being investigated by Reata Pharmaceuticals, Inc. in partnership with Abbott Laboratories and Kyowa Hakko Kirin, as an experimental therapy for advanced chronic kidney disease (CKD) in type 2 diabetes mellitus patients. Reata, in consultation with the BEACON Steering Committee, has decided to terminate the Phase 3 BEACON trial of bardoxolone methyl in patients with stage 4 chronic kidney disease and type 2 diabetes. This decision was made based upon a recommendation of the Independent Data Monitoring Committee (IDMC) to stop the trial “for safety concerns due to excess serious adverse events and mortality in the bardoxolone methyl arm.” [1][2][3][4]
RTA-402 is a triterpenoid anti-inflammatory agent in phase II trials at Reata Pharmaceuticals for the treatment of pulmonary arterial hypertension.
This company and M.D. Anderson Cancer Center had been evaluating clinically the product for the treatment of lymphoma. Reata had been evaluating the compound in combination with gemcitabine in patients with unresectable pancreatic cancer and melanoma. Preclinical studies were also being conducted by Reata for the treatment of inflammatory bowel disease (IBD) and autoimmune disease. Reata Pharmaceuticals and Kyowa Hakko Kirin had been conducting phase II clinical studies for the treatment of diabetic nephropathy. Reata and Abbott also had been conducting phase III clinical trials for delaying progression to end-stage renal disease in patients with chronic kidney disease and type 2 diabetes; however, in 2012 these trials were discontinued due to serious adverse events and mortality. Phase II clinical trials for this indication were discontinued by Kyowa Hakko Kirin in Japan. The compound had been in early clinical studies for the treatment of multiple myeloma; however, no recent development has been reported for this indication. Phase I clinical trials for the treatment of solid tumors have been completed.
RTA-402 has demonstrated a wide variety of potentially therapeutic mechanisms, including inhibition of inducible nitric oxide synthase and cyclooxygenase expression, stimulation of expression of cytoprotective enzymes such as NAD(P)H quinine oxidoreductase and hemeoxygenase-1, and reduction in pSTAT3 levels. In cancer patients, the drug candidate exploits fundamental physiological differences between cancerous and non-cancerous cells by modulating oxidative stress response pathways. Due to this mechanism, RTA-402 is toxic to cancer cells, but induces protective antioxidant and anti-inflammatory responses in normal cells. In previous studies, the compound was shown to inhibit growth and cause regression of cancerous tumors as a single agent and, in combination with radiation and chemotherapy, to suppress radiation and chemotherapy-induced toxicities in normal tissues and cause minimal toxicity in non-human primates when dosed orally at very high doses for 28 consecutive days.
An analog of RTA-401, RTA-402 is a compound found in medicinal plants with a greater potency than the natural product.
RTA-401 was originally developed at Dartmouth College and M.D. Anderson Cancer Center. In November 2004, Reata completed a license agreement with these organizations, and was granted exclusive worldwide rights to this new class of anticancer compounds. In 2008, orphan drug designation was assigned by the FDA for the treatment of pancreatic cancer. In 2010, the compound was licensed to Kyowa Hakko Kirin by Reata Pharmaceuticals in China, Japan, Korea, Thailand and Southeast Asian countries for the treatment of chronic kidney disease. Abbott acquired rights to develop and commercialize the drug outside US, excluding certain Asian markets.
Phase 1
Bardoxolone methyl was first advanced into the clinic to assess its anticancer properties. In two Phase 1 trials that included 81 oncology patients, bardoxolone methyl reduced serum creatinine levels, with a corresponding improvement in estimated glomerular filtration rate (eGFR). Improvements were more pronounced in a subset of patients with established CKD and were maintained over time in patients who continued on bardoxolone methyl therapy for 5 months. Based on these observed effects and the well-described role of oxidative stress and inflammation in CKD, especially in type 2 diabetes, it was hypothesized that bardoxolone methyl could improve renal function in CKD patients with type 2 diabetes.[5]
Phase 2
A multi-center, double-blind, placebo-controlled Phase 2b clinical trial (BEAM) conducted in the US studied 227 patients with moderate to severe CKD (eGFR 20 – 45 ml/min/1.73m²) and type 2 diabetes. The primary endpoint was change in estimated GFR following 24 weeks of treatment. Following 24 weeks, patients treated with bardoxolone methyl experienced a mean increase in estimated GFR of over 10 ml/min/1.73m², compared with no change in the placebo group. Approximately three-quarters of bardoxolone methyl treated patients experienced an improvement in eGFR of 10 percent or more, including one-quarter who saw a significant improvement of 50% or more compared to less than 2% of patients on placebo. Adverse events were generally manageable and mild to moderate in severity. The most frequently reported adverse event in the bardoxolone methyl group was muscle spasm. Final data was published in The New England Journal of Medicine.
Concerns have been raised whether there is a true improvement in kidney function because of the significant weight loss of the patients in the active-treatment-group that ranged from 7.7-10.1 kg (7-10% of the initial body weight) and whether this weight loss in patients receiving bardoxolone included muscle wasting with a commensurate decrease in the serum creatinine level. In that case the decrease in creatinine would not necessarily be a true improvement in kidney function.[6][7][8][9][10]
Phase 3
A multinational, double-blind, placebo-controlled Phase 3 outcomes study (BEACON) was started in June 2011, testing bardoxolone methyl’s impact on progression to ESRD or cardiovascular death in 1600 patients with Stage 4 CKD (eGFR 15 – 30 ml/min/1.73m²) and type 2 diabetes. This phase 3 trail was halted in October 2012 because of adverse effects (namely a higher cardiovascular mortality in the treatment arm).[11]
Mechanism of action
Bardoxolone methyl is an inducer of the KEAP1–Nrf2 pathway.
………………
In a preferred embodiment, such compounds include derivatives of ursolic acid and oleanoic acid. In a particularly preferred embodiment, derivatives of OA, e.g., 2-cyano-3,12-dioxoolean-l,9-dien-28oic acid (CDDO):
have been found to be effective in suppression of human breast cancer cell growth, and highly potent in many vitro assay systems such as: suppression of nitric oxide and prostaglandin production in macrophages, inhibition of growth of human breast cancer cells, suppression of nitric oxide formation in rat prostate cells, and suppression of prostaglandin formation in human colon fibroblasts, as detailed in the Figures.
Compounds were synthesized as below:
Scheme 1
Scheme 2
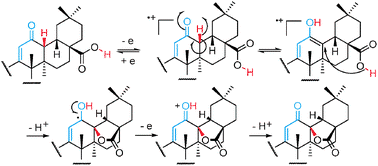
a: HCO2Et/MeONa/THF,b: PhSeCl/AcOEt; 30%H202/THF,c: NH2OH-HCI EtOH/H2O, d: MeONa/MeOH/Et2O,e: KOH/MeOH,f: Jones,g:HCO2Et/MeONa/PhH,h: Lil/DMF Compound 10 was prepared by formylation of OA (Compound 9) (Simonsen and Ross, 1957) with ethyl formate in the presence of sodium methoxide in THF (Clinton et al., 1961). Compound 7 was obtained by introduction of a double bond at C-l of Compound 10 with phenylselenenyl chloride in ethyl acetate and sequential addition of 30%) hydrogen peroxide (Sharpless et al, 1973). Compound 11 was synthesized from Compound 10 by addition of hydroxylamine in aqueous ethanol; cleavage of Compound 11 with sodium methoxide gave Compound 12 (Johnson and Shelberg, 1945). Compound 14 was prepared from Compound 13 (Picard et al, 1939) by alkali hydrolysis followed by Jones oxidation. Compound 15 was prepared by formylation of Compound 14 with ethyl formate in the presence of sodium methoxide in benzene. Compound 16 was synthesized from Compound 15 by addition of hydroxylamine. Cleavage of 16 with sodium methoxide gave Compound 17. Compound 6 (CDDO) was prepared by introduction of a double bond at C-l of Compound 17 with phenylselenenyl chloride in ethyl acetate and sequential addition of 30% hydrogen peroxide, followed by halogenolysis with lithium iodide in DMF (Dean, P.D.G., 1965).
………………………………………………
WO2009/146216 A2,

Compounds 401, 402, 404, 402-04, 402-35 and 402-56 can be prepared according to the methods taught by Honda et al. (1998), Honda et al. (2000b), Honda et al. (2002), Yates et al. (2007), and U.S. Patent 6,974,801, which are all incorporated herein by reference. The synthesis of the other compounds are disclosed in the following applications, each of which is incorporated herein by reference: U.S. Application Nos. 61/046,332, 61/046,342, 61/046,363, 61/046,366, 61/111,333, 61/111,269, and 61/111,294. The synthesis of the other compounds are also disclosed in the following separate applications filed concurrently herewith, each of which is incorporated herein by reference in their entireties: U.S. Patent Application by Eric Anderson, Xin Jiang, Xiaofeng Liu; Melean Visnick, entitled “Antioxidant Inflammation Modulators: Oleanolic Acid Derivatives With Saturation in the C- Ring,” filed April 20, 2009; U.S. Patent Application by Eric Anderson, Xin Jiang and Melean Visnick, entitled “Antioxidant Inflammation Modulators: Oleanolic Acid Derivatives with Amino and Other Modifications At C-17,” filed April 20, 2009; U.S. Patent Application by Xin Jiang, Xioafeng Liu, Jack Greiner, Stephen S. Szucs, Melean Visnick entitled, “Antioxidant Inflammation Modulators: C-17 Homologated Oleanolic Acid Derivatives,” filed April 20, 2009.
………………………………………………
Chemical Communications, 2011 , vol. 47, 33 p. 9495 – 9497
http://pubs.rsc.org/en/Content/ArticleLanding/2011/CC/c1cc11633a#!divAbstract
http://www.rsc.org/suppdata/cc/c1/c1cc11633a/c1cc11633a.pdf NMR GIVEN
2-Cyano-3,12-dioxooleana-1,9(11)-dien-28-oate (CDDO)
A mixture of 1 (0.25 g, 0.51 mmol) and DDQ (0.12 g, 0.51 mmol) in anhydrous benzene (20 mL) was
refluxed for 15 min. After filtration, the filtrate was evaporated in vacuo to give a residue, which was
subjected to flash column chromatography (petroleum ether/EtOAc) to give CDDO as an amorphous
solid (0.23 g, 91%). The title compound was known as CAS 218600-44-3
m.p. 180-182 °C;
ESI-MS: 490 [M-H]-, 492 [M+H]+;
1H NMR (300M Hz, CDCl3, 25 °C, TMS): δ 8.05 (1H, s), 5.99 (1H, s), 3.03-2.98 (2H, m), 1.55,1.38,
1.34, 1.22, 1.00, 0.91, 0.85 (each 3H,s ,CH3) ppm.
………………………..
SYNTHESIS
Journal of Medicinal Chemistry, 2000 , vol. 43, 22 p. 4233 – 4246
http://pubs.acs.org/doi/full/10.1021/jm0002230

Bioorganic and Medicinal Chemistry Letters, 1998 , vol. 8, 19 p. 2711 – 2714
http://www.sciencedirect.com/science/article/pii/S0960894X9800479X

………………………………………………………………………
Bioorganic and Medicinal Chemistry Letters, 2005 , vol. 15, # 9 p. 2215 – 2219
http://www.sciencedirect.com/science/article/pii/S0960894X05003306

………………..
Method of synthesis of CDDO. CDDO may be synthesized by the scheme outlined below.
Methyl-CDDO. Methyl-CDDO (CDDO-Me), the C-28 methyl ester of CDDO, also exerts strong antiproliferative and apoptotic effects on leukemic cell lines and in primary AML samples in vitro as well as induces monocytic differentiation of leukemic cell lines and some primary AMLs. Thus, CDDO-Me provides chemotherapy for the treatment of leukemias. The present invention demonstrates that this effect is profoundly increased by combination of CDDO-Me with other chemotherapeutic agents. These include retinoids such as ATRA, 9-cis retinoic acid, , LG100268, LGD1069 (Targretin, bexarotene), fenretinide [N-(4- hydroxyphenyl)retinamide, 4-HPR], CD437 and other RXR and RAR-specific ligands. This combination also increases ara-C cytotoxicity, further reduces AML colony formation, inhibits ERK phosphorylation and promotes Bcl-2 dephosphorylation, and inhibits in vitro angiogenesis. The ability of CDDO-Me in combination with retinoids to induce differentiation in leukemic cells in vitro show that these compounds may have similar in vivo effects. The anti-angiogenic properties of CDDO-Me further increase its potent anti-leukemia activity in combination with retinoids. Furthermore, CDDO-Me was found to be more potent at lower concentrations than CDDO.
Method of synthesis of CDDO-Me.
CDDO-Me may be synthesized by the scheme outlined below.
The present invention provides combinations of CDDO-compounds and chemotherapeutic agents that are useful as treatments for cancers and hematological malignancies. In one embodiment, the chemotherapeutics are retinoids. As CDDO- compounds are PPARγ ligands and PPARγ is known to be altered in many types of cancers, the inventors contemplate, that ligation of PPARγ in combination with retinoids such as, RXR-specific ligands, provides a mechanistic basis for maximal increase in transcriptional activity of the target genes that control apoptosis and differentiation. The CDDO-compounds and retinoids in combination demonstrate an increased ability to induce differentiation, induce cytotoxicity, induce apoptosis, induce cell killing, reduce colony formation and inhibit the growth of several types of leukemic cells.
…………………..
Efficient and scalable synthesis of bardoxolone methyl (cddo-methyl ester).
Bardoxolone methyl (2-cyano-3,12-dioxooleane-1,9(11)-dien-28-oic acid methyl ester; CDDO-Me) (1), a synthetic oleanane triterpenoid with highly potent anti-inflammatory activity (levels below 1 nM), has completed a successful phase I clinical trial for the treatment of cancer and a successful phase II trial for the treatment of chronic kidney disease in type 2 diabetes patients. Our synthesis of bardoxolone methyl (1) proceeds in ∼50% overall yield in five steps from oleanolic acid (2), requires only one to two chromatographic purifications, and can provide gram quantities of 1.
References
- “Bardoxolone methyl – Oral, Once Daily AIM for Renal/Cardiovascular/Metabolic Diseases”. Reata Pharmaceuticals. Archived from the original on 15 July 2011. Retrieved June 2, 2011.
- “Abbott and Reata Pharmaceuticals Announce Agreement to Develop and Commercialize Bardoxolone Methyl for Chronic Kidney Disease Outside the U.S.” (Press release). Reata Pharmaceuticals. September 23, 2010. Retrieved June 2, 2011.
- “Reata Pharmaceuticals Licenses Chronic Kidney Disease Drug Bardoxolone Methyl to Kyowa Hakko Kirin”(Press release). Reata Pharmaceuticals. January 7, 2010. Retrieved June 2, 2011.
- “Company Statement: Termination of Beacon Trial”.Reata Pharmaceuticals. Retrieved October 18, 2012.
- Pergola, P. E.; Krauth, M.; Huff, J. W.; Ferguson, D. A.; Ruiz, S.; Meyer, C. J.; Warnock, D. G. (2011). “Effect of Bardoxolone Methyl on Kidney Function in Patients with T2D and Stage 3b–4 CKD”. American Journal of Nephrology 33 (5): 469–476. doi:10.1159/000327599. PMID 21508635.
- Pergola, P. E.; Raskin, P.; Toto, R. D.; Meyer, C. J.; Huff, J. W.; Grossman, E. B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; Wittes, J.; Warnock, D. G.; Beam Study, I. (2011). “Bardoxolone Methyl and Kidney Function in CKD with Type 2 Diabetes” (pdf). New England Journal of Medicine 365 (4): 327–336.doi:10.1056/NEJMoa1105351. PMID 21699484. edit
- van Laecke, S.; Vanholder, R. (2011). “Communication: Bardoxolone methyl, chronic kidney disease, and type 2 diabetes”. New England Journal of Medicine 365 (18): 1745, author reply 1746–1747.doi:10.1056/NEJMc1110239. PMID 22047578.
- Rogacev, K. S.; Bittenbring, J. T.; Fliser, D. (2011).“Communication: Bardoxolone methyl, chronic kidney disease, and type 2 diabetes”. New England Journal of Medicine 365 (18): 1745–1746, author reply 1746–1747.doi:10.1056/NEJMc1110239. PMID 22047579.
- Upadhyay, A.; Sarnak, M. J.; Levey, A. S. (2011).“Communication: Bardoxolone methyl, chronic kidney disease, and type 2 diabetes”. New England Journal of Medicine 365 (18): 1746, author reply 1746–1747.doi:10.1056/NEJMc1110239. PMID 22047580.
- McMahon, G. M.; Forman, J. P. (2011). “Communication: Bardoxolone methyl, chronic kidney disease, and type 2 diabetes”. New England Journal of Medicine 365 (18): 1746, author reply 1746–1747.doi:10.1056/NEJMc1110239. PMID 22047581.
- ClinicalTrials.gov NCT01351675 Bardoxolone Methyl Evaluation in Patients With Chronic Kidney Disease and Type 2 Diabetes (BEACON)
- Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages
Bioorg Med Chem Lett 1998, 8(19): 2711 - Novel synthetic oleanate triterpenoids: A series of highly active inhibitors of nitric production in mouse macrophages
Bioorg Med Chem Lett 1999, 9(24): 3429 - WO 1999065478
- WO 2013169553
- CN 102875634
- US 2012330050
- US 2012071684
- WO 2011130302
- WO 2010093944
- WO 2009089545
- WO 2009023232
- WO 2008111497
- Anderson, Amy C.; Browning, R. Greg; Couch, Robin D.; Gribble, Gordon W.; Honda, Tadashi; Wright, Dennis L.; Sporn, Michael B.
Bioorganic and Medicinal Chemistry Letters, 2005 , vol. 15, 9 p. 2215 – 2219 - Journal of Medicinal Chemistry, 2004 , vol. 47, 20 p. 4923 – 4932
- Journal of Medicinal Chemistry, 2000 , vol. 43, 22 p. 4233 – 4246
- Bioorganic and Medicinal Chemistry Letters, 2002 , vol. 12, 7 p. 1027 – 1030
- Journal of Medicinal Chemistry, 2000 , vol. 43, 22 p. 4233 – 4246
- Chemical Communications, 2011 , vol. 47, 33 p. 9495 – 9497
- Reata Pharmaceuticals, Inc. – bardoxolone methyl
- Bardoxolone Methyl and Kidney Function in CKD with Type 2 Diabetes
- Effect of Bardoxolone Methyl on Kidney Function in Patients with T2D and Stage 3b – 4 CKD
- American Diabetes Association presentation
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US8440854 * | Jan 23, 2012 | May 14, 2013 | Reata Pharmaceuticals, Inc. | Antioxidant inflammation modulators: oleanolic acid derivatives with amino acid and other modifications at C-17 |
| US8513436 | Dec 19, 2011 | Aug 20, 2013 | Reata Pharmaceuticals, Inc. | Pyrazolyl and pyrimidinyl tricyclic enones as antioxidant inflammation modulators |
| WO2002047611A2 * | Nov 28, 2001 | Jun 20, 2002 | Univ Texas | Cddo-compounds and combination therapies thereof |
| WO2008064132A2 * | Nov 16, 2007 | May 29, 2008 | Dartmouth College | Synthetic triterpenoids and tricyclic-bis-enones for use in stimulating bone and cartilage growth |
| WO2009118441A1 * | Feb 12, 2009 | Oct 1, 2009 | Consejo Superior De Investigaciones Cientifícas | Use of pentacyclic triterpene for the preparation of a pharmaceutical compound intended for the treatment of multiple sclerosis |
| WO2013083659A1 | Dec 5, 2012 | Jun 13, 2013 | Cambridge Enterprise Limited | Combination treatment comprising ho – 1 inhibitor and immunotherapeutic agent |
| US7176237 | Jan 15, 2003 | Feb 13, 2007 | The Trustees Of Dartmouth College | Tricyclic-bis-enone derivatives and methods of use thereof |
| US7435755 | Nov 28, 2001 | Oct 14, 2008 | The Trustees Of Dartmouth College | CDDO-compounds and combination therapies thereof |
| US7678830 | Feb 7, 2007 | Mar 16, 2010 | Trustees Of Dartmouth College | Tricyclic-bis-enone derivatives and methods of use thereof |
| US7714012 | Nov 16, 2007 | May 11, 2010 | Trustees Of Dartmouth University | Synthesis and biological activities of new tricyclic-bis-enones (TBEs) |
| US7795305 | Oct 10, 2008 | Sep 14, 2010 | Board Of Regents, The University Of Texas System | CDDO-compounds and combination therapies thereof |
| US7863327 | May 3, 2005 | Jan 4, 2011 | Trustees Of Dartmouth College | Therapeutic compounds and methods of use |
| US7915402 | Apr 20, 2009 | Mar 29, 2011 | Reata Pharmaceuticals, Inc. | Antioxidant inflammation modulators: oleanolic acid derivatives with saturation in the C-ring |
| US7943778 | Apr 20, 2009 | May 17, 2011 | Reata Pharmaceuticals, Inc. | Antioxidant inflammation modulators: C-17 homologated oleanolic acid derivatives |
| US8034955 | Oct 29, 2007 | Oct 11, 2011 | Trustees Of Dartmouth College | Therapeutic compounds and methods of use |
| US8067394 | May 10, 2010 | Nov 29, 2011 | Trustees Of Dartmouth College | Synthesis and biological activities of new tricyclic-bis-enones (TBEs) |
| US8067465 | Mar 11, 2010 | Nov 29, 2011 | The Trustees Of Dartmouth College | Tricyclic-bis-enone derivatives and methods of use thereof |
| US8071632 | Apr 20, 2009 | Dec 6, 2011 | Reata Pharmaceuticals, Inc. | Antioxidant inflammation modulators: novel derivatives of oleanolic acid |
| US8124656 | Feb 23, 2011 | Feb 28, 2012 | Reata Pharmaceuticals, Inc. | Antioxidant inflammation modulators: oleanolic acid derivatives with saturation in the C-ring |
| US8124799 | Apr 20, 2009 | Feb 28, 2012 | Reata Pharmaceuticals, Inc. | Antioxidant inflammation modulators: oleanolic acid derivatives with amino and other modifications at C-17 |
| US8129429 | Jan 12, 2009 | Mar 6, 2012 | Reata Pharmaceuticals, Inc. | Synthetic triterpenoids and methods of use in the treatment of disease |
| US8258329 | Apr 20, 2009 | Sep 4, 2012 | Reata Pharmaceuticals, Inc. | Dehydroandrosterone analogs including an anti-inflammatory pharmacore and methods of use |
| US8299046 | Nov 16, 2007 | Oct 30, 2012 | Trustees Of Dartmouth College | Synthetic triterpenoids and tricyclic-bis-enones for use in stimulating bone and cartilage growth |
| US8314137 | Jul 22, 2009 | Nov 20, 2012 | Trustess Of Dartmouth College | Monocyclic cyanoenones and methods of use thereof |
| US8338618 | Nov 11, 2011 | Dec 25, 2012 | Reata Pharmaceuticals, Inc. | Antioxidant inflammation modulators: novel derivatives of oleanolic acid |
| US8394967 | Feb 23, 2011 | Mar 12, 2013 | Reata Pharmaceuticals, Inc. | Antioxidant inflammation modulators: C-17 homologated oleanolic acid derivatives |
| US8440820 | Jan 11, 2012 | May 14, 2013 | Reata Pharmaceuticals, Inc. | Antioxidant inflammation modulators: oleanolic acid derivatives with saturation in the C-ring |
| US8440854 | Jan 23, 2012 | May 14, 2013 | Reata Pharmaceuticals, Inc. | Antioxidant inflammation modulators: oleanolic acid derivatives with amino acid and other modifications at C-17 |
| US8455544 | Jan 26, 2012 | Jun 4, 2013 | Reata Pharmaecuticals, Inc. | Synthetic triterpenoids and methods of use in the treatment of disease |
| US8513436 | Dec 19, 2011 | Aug 20, 2013 | Reata Pharmaceuticals, Inc. | Pyrazolyl and pyrimidinyl tricyclic enones as antioxidant inflammation modulators |
| US8586775 | Aug 24, 2011 | Nov 19, 2013 | Trustees Of Dartmouth College | Therapeutic compounds and methods of use |
 |
| Professor Honda received his B.S. degree in Chemistry in 1974, his M.S. degree in Organic Chemistry in 1976, and his Ph.D. in Organic Chemistry in 1979 from the University of Tokyo. In 1979, he joined the Department of Drug Discovery Chemistry at Suntory Institute for Biomedical Research in Japan and worked there as a drug synthetic chemist (finally senior researcher) for 13 years. In 1991, he joined the Central Pharmaceutical Research Institute at Japan Tobacco Inc. and worked as a chief senior researcher for 3 years. In 1995, he joined Dr. Gribble’s laboratory at Dartmouth College as a research associate. In 1998, he joined the research faculty of Dartmouth College. In 2005, he was promoted to Research Associate Professor.http://www.dartmouth.edu/~chem/faculty/th.html |
Dr. Honda and his collaborators have further explored new structures based on CDDO and different five-ringed triterpenoids.
During the course of these investigations, Dr. Honda has designed three-ringed compounds with similar enone functionalities in rings A and C to those of CDDO, but having a much simpler structure than five-ringed triterpenoids. He and his collaborators have found that they are also a novel class of potent anti-inflammatory, cytoprotective, growth suppressive, and pro-apoptotic compounds. Amongst such three-ringed compounds, TBE-31 with the C-8a ethynyl group is much more potent than CDDO in various bioassays in vitro and in vivo. Thus, further investigation (design, synthesis, biological evaluation, etc.) of new TBE-31 analogues is currently being performed in order to discover analogues having different and/or better features than TBE-31, for example, higher potency and lower toxicity, better bioavailability and different distributions in organs, high water-solubility and so on.

Mechanism studies suggest that CDDO regulates various molecules regarding inflammation, differentiation, apoptosis, and proliferation by reversible Michael addition between the cyano enone functionality of CDDO and the sulfhydryl groups of cysteine moieties on these molecules. Based on this fact and the structure of TBE-31, Dr. Honda has designed single-ringed compounds, which represent the ideal simple structure. The synthesis of these new compounds is currently in progress.