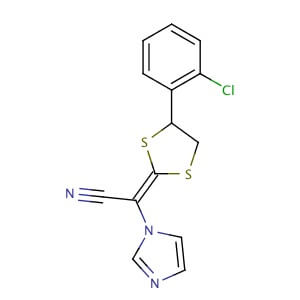
Lanoconazole
READ AT http://newdrugapprovals.org/2014/10/02/lanoconazole/
- Latoconazole, Lanoconazole, TJN-318, NND-318, Astat,
Nihon Nohyaku (Originator), Tsumura (Licensee)
| Synonym: | 2-[4-(2-Chlorophenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-yl-acetonitrile |
| Application: | An antifungal compound |
| CAS Number: | 101530-10-3 |
| Molecular Weight: | 319.83 |
| Molecular Formula: | C14H10ClN3S2 |
Brief background information
| Appearance: | Crystalline |
| Physical State: | Solid |
| Solubility: | Soluble in chloroform, and methanol. Insoluble in water. |
| Storage: | Store at -20° C |
| Melting Point: | 129-132 °C |
| Boiling Point: | ~477.6 °C at 760 mmHg (Predicted) |
| Density: | ~1.4 g/cm3 (Predicted) |
| Refractive Index: | n20D 1.73 (Predicted) |
| pK Values: | pKb: 3.76 (Predicted) |
| WGK Germany: | 3 |
| RTECS: | NI3393500 |
| PubChem CID: | 3002820 |
| Merck Index: | 14: 5357 |
| MDL Number: | MFCD00865590 |
| Beilstein Registry: | 4819111 |
| Salt | ATC | Formula | MM | CAS |
|---|---|---|---|---|
| – | D01 | C 14 H 10 ClN 3 S 2 | 319.84 g / mol | 101530-10-3 |

Application
-
antifungal
Synthesis pathway
| Synthesis a) |
|---|
  |
Trade Names
| Country | Trade name | Manufacturer |
|---|---|---|
| Japan | Astatine | Tsumura |
| Ukraine | No | No |
Formulations
-
1% cream;
-
1% ointment;
-
1% solution
Links
-
EP 218 736 (Nihon Nohyaku; EP-prior. 9.10.1985).
1. Oka, H., et al., 1992. Therapeutic efficacy of latoconazole in formulations of clinical use on experimental dermatophytosis in guinea pigs. Arzneimittel-Forschung. 42(3): 345-9. PMID: 1497697
2. Niwano, Y., et al., 1994. Therapeutic efficacy of lanoconazole, a new imidazole antimycotic agent, for experimental cutaneous candidiasis in guinea pigs. Antimicrobial agents and chemotherapy. 38(9): 2204-6. PMID: 7811048
3 http://aac.asm.org/content/38/9/2204.full.pdf
References 1. Seo, A., Kanno, H., Hasegawa, N. et al. (Nihon Nohyaku Co., Ltd.). Antimycotic agent and fungicidal agent. US 4738976. 2. Seo, A ., Sugano, H., Hasegawa, C., Ikeda, K., Munechica, Y., Konoe, T., Konaka, M. (Nihon Nohyaku Co., Ltd.). Antifungal agent. JP 87093227. 3. Seo , A., Sugano, H., Hasegawa, C., Ikeda, K., Nishimura, A., Miyashiro, Y. (Nihon Nohyaku Co., Ltd.). Non-medicinal bactericidal agents and method for their preparation. JP 87093204. 4. Seo, A., Sugano, H., Hasegawa, C., Miyashiro, Y., Nishimura, A., Ikeda, K. (Nihon Nohyaku Co., Ltd.). Ketene S, S-acetals. JP 85218387. 5. Seo, A., Kanno, H., Hasegawa, N. et al. (Nihon Nohyaku Co., Ltd.). A novel ketene S, S-acetal deriv., a process for manufacturing thereof and a method for curing mycosis by administering it. EP 218736.
MAKE IN INDIA
http://makeinindia.com/sector/pharmaceuticals/
Read all about Organic Spectroscopy on ORGANIC SPECTROSCOPY INTERNATIONAL