COSY PREDICT
COSY PREDICT
1H NMR PREDICT
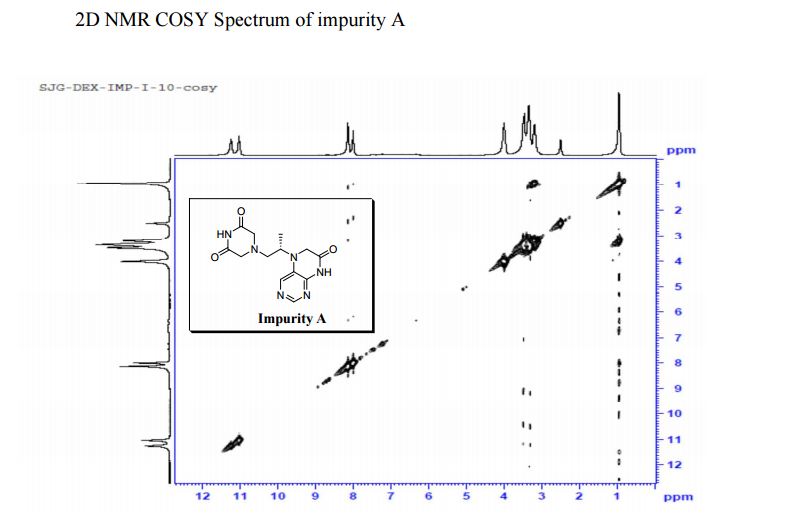
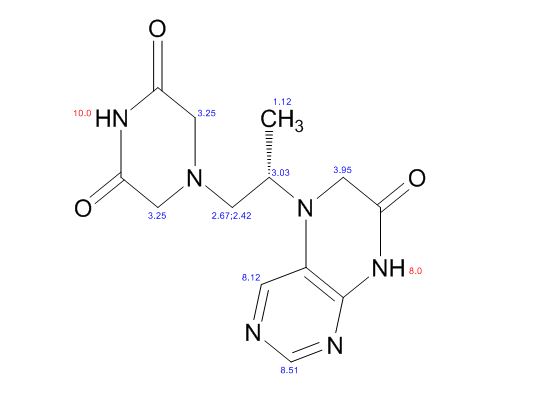
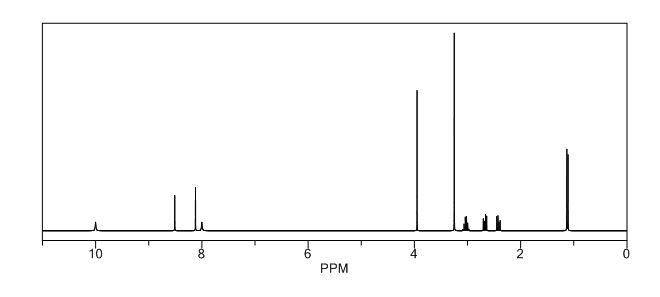
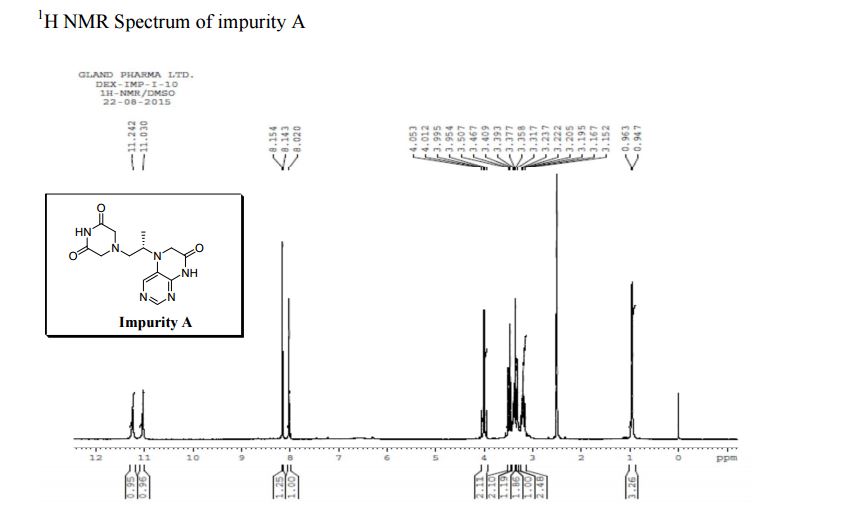
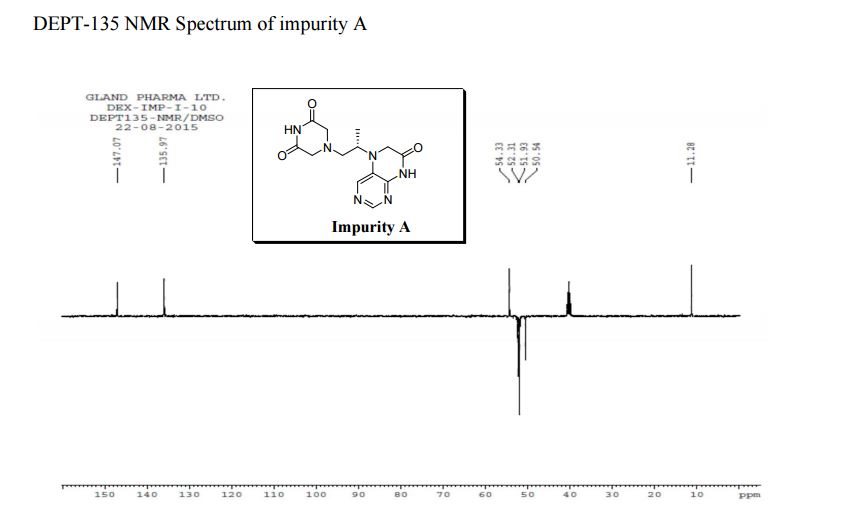
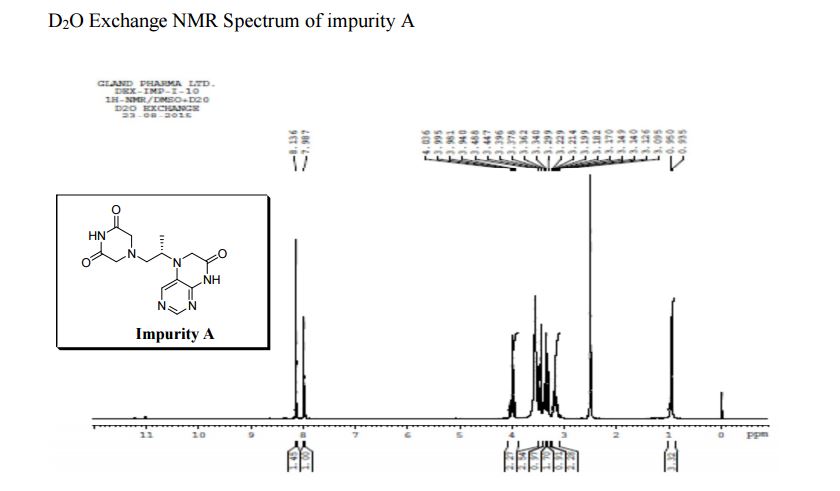
1H NMR (DMSO-d6, 400 MHz): δH 0.95 (3H, d, H4), 3.09–3.23 (2H, m, H2), 3.29–3.49 (5H, m, H14, H11, H3), 3.94–4.04 (2H, m, H5), 8.14 (1H, s, H8), 8.02 (1H, s, H9).
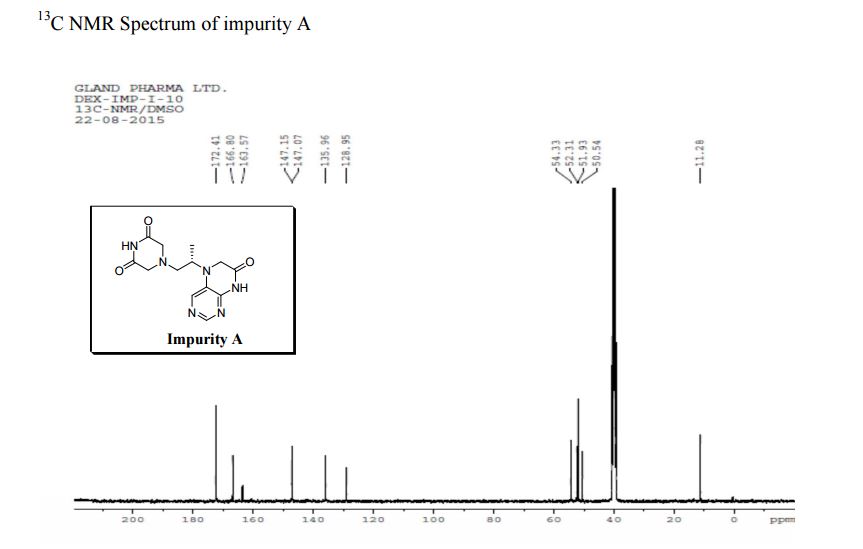
13C NMR PREDICT

Houben-Weyl methods of organic chemistry, 4th ed.; Vol. E 9c Hetarenes III part 3; p 279.
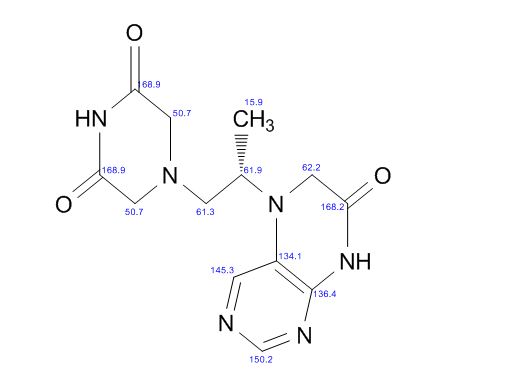
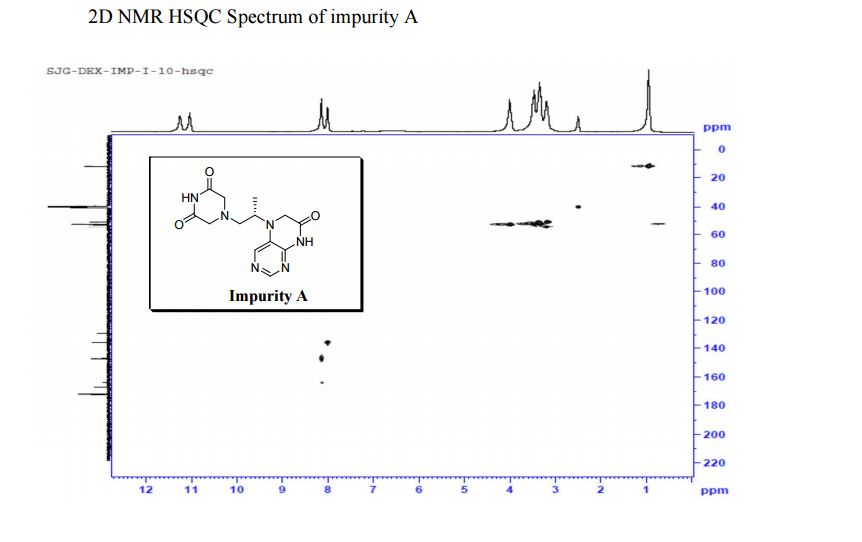
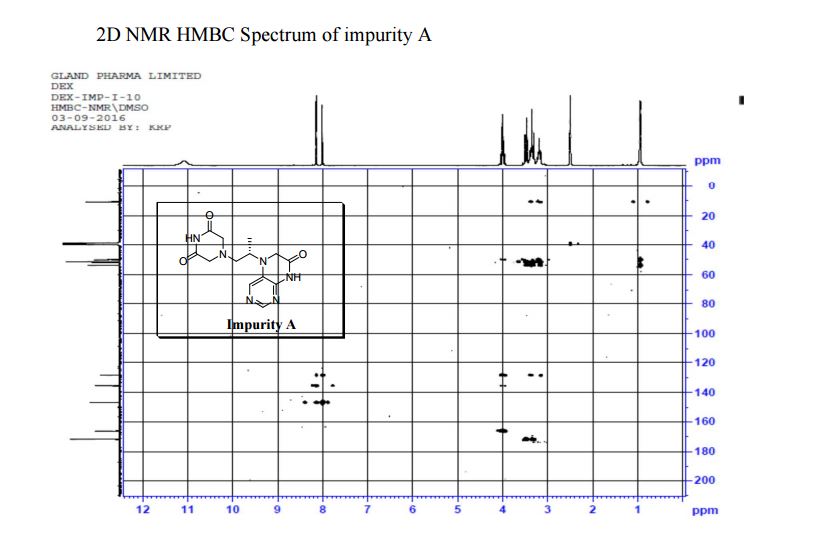
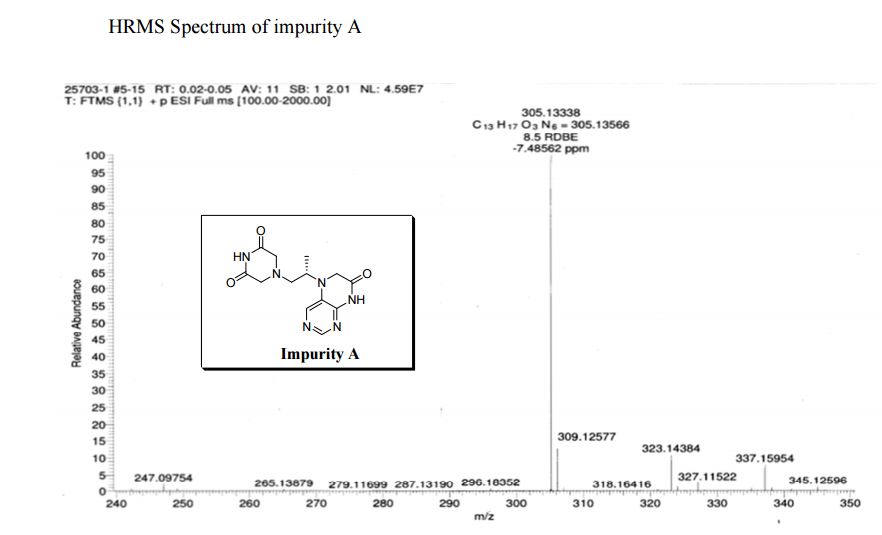
5-(2-(3-Oxopiperazin-1-yl) propyl)-5,6-dihydropteridin-7(8H)-one (Impurity A)
Study on the Isolation and Chemical Investigation of Potential Impurities in Dexrazoxane Using 2D-NMR and LC-PDA-MS



A chemical investigation of process related impurities associated with the synthesis of dexrazoxane was performed. The degradation product of dexrazoxane is known in the literature; however, the information related to process impurities is not available. For the first time, two process related impurities were isolated, characterized, and confirmed by their individual chemical synthesis. The present study describes the isolation methods of the impurities and their structural elucidation using IR, 1H, 13C, 2D NMR, and mass spectrometry. The identification of these impurities would be highly valuable for the quality control during the production of the dexrazoxane drug substance
The U.S. Food and Drug Administration (FDA)(5) and the European Medicine Agency (EMA)(6) require analytical characterization not only for the active pharmaceutical ingredient (API), but also for its key starting materials and advanced intermediates. The determination of a drug substance impurity profile, including especially known pharmacopeial impurities, as well as other unknown impurities, could have a significant impact on the quality and the safety of the drug products. The health implications of the impurities could be significant because of their potential mutagenic or carcinogenic effects. Therefore, the International Conference on Harmonization (ICH) has set a high standard for the purity of drug substances.(7) If the dose is less than 2 g/day, then impurities over 0.10% are expected to be identified, qualified, and controlled. If the dose exceeds 2 g/day, then the qualification threshold is lowered to 0.05%. It is therefore essential to monitor and control the impurities in both the drug substance (API) and the drug products.
-
5 Guidance for Industry on Abbreviated New Drug Applications: Impurities in Drug Substances; Availability;Fed. Regist. 2009, 74, 34359– 34360.
-
6.The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Impurities in New Drug Substances Q3A (R2); IGH: Geneva, Switzerland, 2006.
-
7.International Conference on Harmonization; revised guidance on Q3A impurities in new drug Substances; Availability; Fed. Regist. 2003, 68, 6924– 6925.
//////////5-(2-(3-Oxopiperazin-1-yl) propyl)-5,6-dihydropteridin-7(8H)-one
O=C1Nc3ncncc3N(C1)[C@@H](C)CN2CC(=O)NC(=O)C2