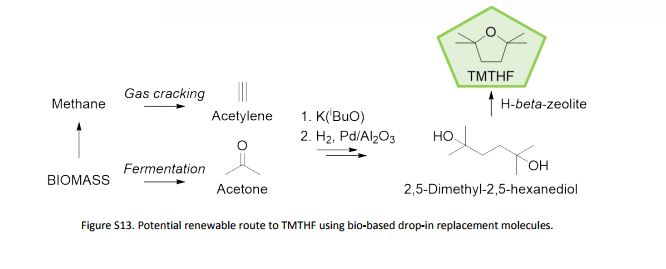
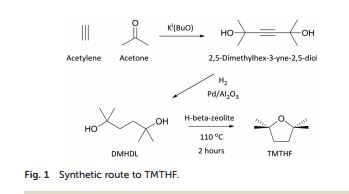
2,2,5,5-Tetramethyltetrahydrofuran (TMTHF): a non-polar, non-peroxide forming ether replacement for hazardous hydrocarbon solvents
DOI: 10.1039/C7GC01392B, Paper
An inherently non-peroxide forming ether solvent, 2,2,5,5-tetramethyltetrahydrofuran (2,2,5,5-tetramethyloxolane), has been synthesized from readily available and potentially renewable feedstocks, and its solvation properties have been tested

- From the journal:
Green Chemistry
2,2,5,5-Tetramethyltetrahydrofuran (TMTHF): a non-polar, non-peroxide forming ether replacement for hazardous hydrocarbon solvents
Abstract
An inherently non-peroxide forming ether solvent, 2,2,5,5-tetramethyltetrahydrofuran (2,2,5,5-tetramethyloxolane), has been synthesized from readily available and potentially renewable feedstocks, and its solvation properties have been tested. Unlike traditional ethers, its absence of a proton at the alpha-position to the oxygen of the ether eliminates the potential to form hazardous peroxides. Additionally, this unusual structure leads to lower basicity compared with many traditional ethers, due to the concealment of the ethereal oxygen by four bulky methyl groups at the alpha-position. As such, this molecule exhibits similar solvent properties to common hydrocarbon solvents, particularly toluene. Its solvent properties have been proved by testing its performance in Fischer esterification, amidation and Grignard reactions. TMTHF’s differences from traditional ethers is further demonstrated by its ability to produce high molecular weight radical-initiated polymers for use as pressure-sensitive adhesives.
[TMTHF].
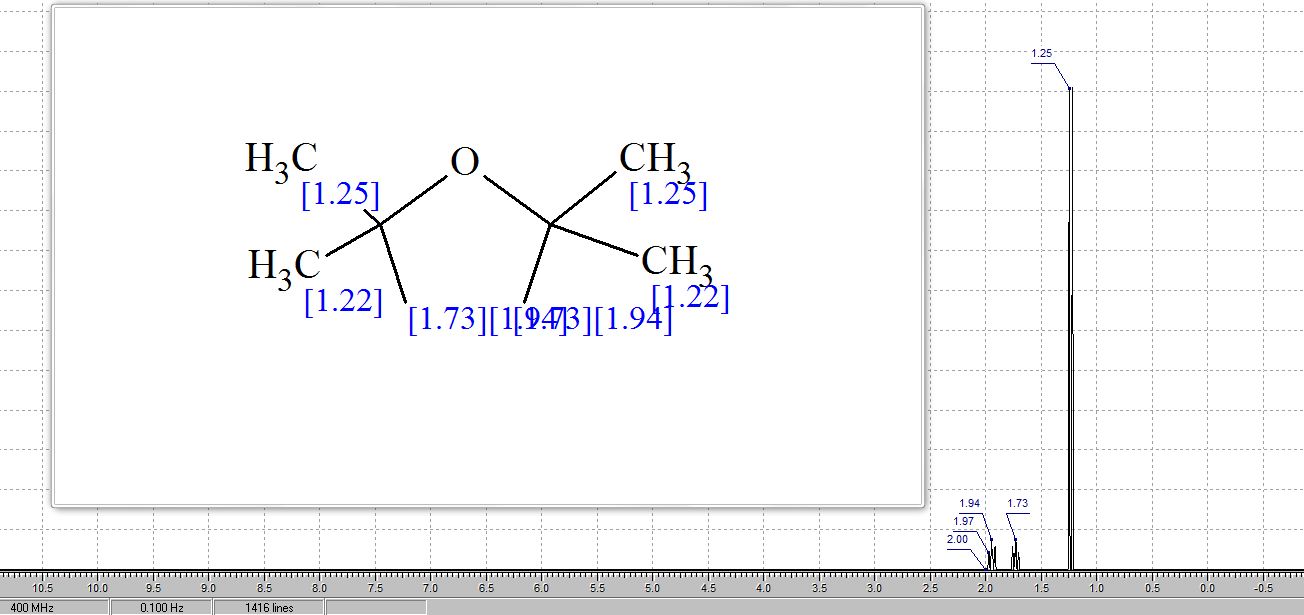
1H NMR (400 MHz, CDCl3): δ 1.81 (s, 4H), 1.21 (s, 12H);
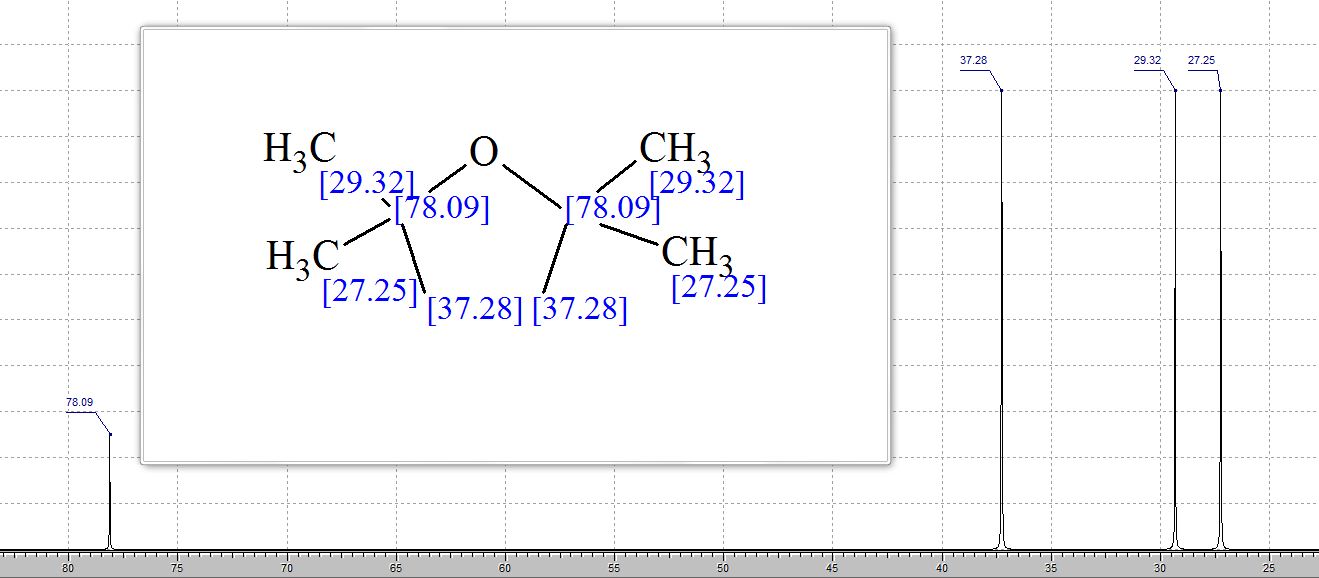
13C NMR (400 MHz, CDCl3): δ 29.75, 38.75, 80.75;
IR 2968, 2930, 2968, 1458, 1377, 1366, 1310, 1265, 1205, 1144, 991, 984, 885, 849, 767 cm−1;
m/z (%): (ESI–MS) 128 (40) [M+ ]

Fergal Byrne
PHD Researcher at Green Chemistry Centre of Excellence
University of York
York, United Kingdom

Green Chemistry Centre of Excellence, University of York, York YO10 5DD, UK

Andrew Hunt
Catalysis, Environmental Chemistry, Green Chemistry
////////////
NMR predict
[TMTHF].
1H NMR (400 MHz, CDCl3): δ 1.81 (s, 4H), 1.21 (s, 12H);
13C NMR (400 MHz, CDCl3): δ 29.75, 38.75, 80.75;