Pexidartinib
PLX-3397
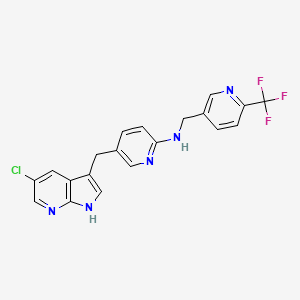
5-((5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl)-N-((6-(trifluoromethyl)pyridin-3-yl)methyl)pyridin-2-amine
N-[5-[(5-Chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl]-2-pyridinyl]-6-(trifluoromethyl)-3-pyridinemethanamine
Phase III
A Multi-targeted tyrosine kinase inhibitor potentially for the treatment of tenosynovial giant cell tumor (TGCT).
![]()
| CAS No.: | 1029044-16-3 |
| Mol. Formula: | C20H15ClF3N5 |
| Mol. Weight: | 417.81 |
- Pexidartinib; 1029044-16-3; PLX-3397; 5-((5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl)-N-((6-(trifluoromethyl)pyridin-3-yl)methyl)pyridin-2-amine; 5-[(5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl]-N-[[6-(trifluoromethyl)pyridin-3-yl]methyl]pyridin-2-amine; 5-[(5-Chloro-1h-Pyrrolo[2,3-B]pyridin-3-Yl)methyl]-N-{[6-(Trifluoromethyl)pyridin-3-Yl]methyl}pyridin-2-Amine;
- Originator Plexxikon
- Developer Barbara Ann Karmanos Cancer Institute; Columbia University; Merck & Co; National Cancer Institute (USA); Plexxikon; University of California at San Francisco
- Class 2 ring heterocyclic compounds; Antineoplastics; Fluorine compounds; Pyridines; Pyrroles; Small molecules
- Mechanism of Action Fms-like tyrosine kinase 3 inhibitors; Immunomodulators; Macrophage colony stimulating factor receptor antagonists; Proto oncogene protein c-akt inhibitors; Proto oncogene protein c-kit inhibitors
- Orphan Drug Status Yes – Giant cell tumour of tendon sheath; Pigmented villonodular synovitis
- Phase III Pigmented villonodular synovitis
- Phase II Glioblastoma; Malignant melanoma; Prostate cancer
- Phase I/II Breast cancer; Leukaemia; Peripheral nervous system diseases; Sarcoma; Solid tumours
- Phase I Gastrointestinal stromal tumours
- No development reported Neurological disorders; Rheumatoid arthritis
- Discontinued Acute myeloid leukaemia; Hodgkin’s disease
Most Recent Events
- 25 May 2016 Plexxikon and AstraZeneca plan the MEDIPLEX phase I trial for Solid tumours (Combination therapy, Metastatic disease) in France (NCT02777710)
- 05 Apr 2016 Daiichi Sankyo plans a phase I trial for Solid tumours (Late-stage disease, Second-line therapy or greater) in Taiwan (PO) (NCT02734433)
- 11 Mar 2016 Plexxikon re-initiates enrolment in a phase Ib trial in Solid tumours and Gastrointestinal stromal tumours in USA (NCT02401815)

Multi-targeted receptor tyrosine kinase inhibitor of CSF1R, c-Kit, and FLT3 (IC50 values 13 nM, 27 nM, and 11 nM, respectively) Administration of PLX3397 reduced CIBP, induced substantial intratumoral fibrosis, and was also highly efficacious in reducing tumor cell growth, formation of new tumor colonies in bone, and pathological tumor-induced bone remodeling. PLX3397 is superior to imatinib in the treatment of malignant peripheral nerve sheath tumor (MPNST), and the combination of PLX3397 with a TORC1 inhibitor could provide a new therapeutic approach for the treatment of this disease.
Plexxikon is conducting phase III clinical studies with PLX-3397 for the treatment of pigmented villonodular synovitis. Phase II clinical studies are ongoing for the oral treatment of melanoma and glioblastoma multiforme. Additional early clinical trials are underway for the treatment of metastatic breast cancer, for the treatment of prostate cancer (adenocarcinoma), and for the treatment of malignant peripheral nerve sheath tumor. No recent development has been reported from preclinical studies for the treatment of systemic lupus erythematosus and for the treatment of multiple sclerosis. Prior to patient enrollment, a phase I clinical trial by Plexxikon for the treatment of rheumatoid arthritis was withdrawn. Daiichi Sankyo (parent of Plexxikon) decided to discontinue phase II trials of the product for the treatment of castration-resistant prostate cancer and for the treatment of Hodgkin’s lymphoma after reviewing its clinical study results and also have discontinued phase II studies for the treatment of acute myeloid leukemia due to strategic reasons.
In 2014, orphan drug designation was assigned to the compound in the US for the treatment of pigmented villonodular synovitis andf giant cell tumor of the tendon sheath. In 2015, the compound was granted orphan designation in the E.U. for the treatment of tenosynovial giant cell tumor, localised and diffuse type. In the same year, the product was granted breakthrough therapy designation for the treatment of tenosynovial giant cell tumor (TGCT) where surgical removal of the tumor would be associated with potentially worsening functional limitation or severe morbidity.
C-fms and c-kit arc both type III transmembrane receptor protein tyrosine kinases (RPTKs) that regulate key signal transduction cascades that control cellular growth and proliferation. Both receptors have similar structural features comprising five extracellular immunoglobulin (IG) domains, a single transmembrane domain, and a split cytoplasmic kinase domain separated by a kinase insert segment.
c-Fms
C-fms is a member of the family of genes originally isolated from the Susan McDonough strain ot teline sarcoma viruses, The cellular proto-oncogene FMS (c-fms, cellular feline McDonough sarcoma) codes for the receptor for the macrophage colony-stimuktmg tactor (M- CSF) C-fms is crucial for the growth and differentiation of the monocyte-macrophage lineage, and upon binding of Vf-CSF to the extracellular domain of c-fms, the receptor dimeπzes and trans- autophosphorylates cytoplasmic tyrosine residues
M-CSF, first described by Robinson and co-workers (Blood 1969, 33 396-9), is a cytokine that controls the production, differentiation, and function of macrophages M-CSF stimulates differentiation of progenitor cells to mature monocytes, and prolongs the survival of monocytes Furthermore, M-CSF enhances cytotoxicity, superoxide production, phagocytosis, chemota\is, and secondary cytokine production of additional factors in monocytes and macrophages Examples of such additional factors include granulocyte colony stimulating lactor (G-CSF) interleukin-6 (IL-6), and mterleukm-8 (IL-8) M-CSF stimulates hematopoiesis, promotes differentiation and proliferation of osteoclast progenitor cells, and has profound effects on lipid metabolism Furthermore, M-CSF is important in pregnancy Physiologically, large amounts of M-CSF are produced in the placenta, and M-CSF is believed to play an essential role in trophoblast differentiation (Motoyoshi, lnt J Hematol 1998, 67 109-22) l hc elevated semm levels of M-CSF m early pregnancy may participate in the immunologic mechanisms responsible for the maintenance of the pregnancy (Flanagan & Lader, Curr Opm Hematol 1998, 5 181-5)
Related to c-fms and c-kit are two p_latelet -derived growth factor receptors, alpha (i e , pdgfra) and beta (pdgfrb) (PDGF) 1 he gene coding for pdgfra is located on chromosome 4ql 1 -q!2 in the same region of chromosome 4 as the oncogene coding for c-kit The genes coding for pdgfra and c-fms appear to have evolved from a common ancestral gene by gene duplication, inasmuch as these two genes are tandemly linked on chromosome 5 They are oriented head to tail with the 5-pnme exon of the c-fms gene located only 500 bp from the last 3-pπme exon of the gene coding for pdgfra Most gastrointestinal stromal tumors (GIST) have activating mutations in c-kit and most patients with GISTs respond well to Gleevec, which inhibits c-kit Hemπch et al (Science 2003, 299 “OS-IO) have shown that approximately 35% of GISTs lacking c-krt mutations, have intragenic activation mutations m tht gene encoding pdgfra, and that tumors expressing c-kit or pdgfrd are indistinguishable with respect to activation of downstream signaling intermediates and cytogenetic changes associated with tumor progression Thus, c kit and pdgfra mutations appear to be alternative and mutually exclusive oncogenic mechanisms m GISTs [0007} Similarly, the observation that production of M-CSF, the major macrophage growth factor, is increased in tissues during inflammation points out a role for c-frns in diseases, such as for example inflammatory diseases. More particularly, because elevated levels of M-CSF are found in the disease state, modulation of the activity of c-fms can ameliorate disease associated with increased levels of M-CSF.
c-Kit
The Stem Cell Factor (SCF) receptor c-kit plays an important role in the development of melanocytes and mast, germ and hematopoietic cells. Stem Cell Factor (SCF) is a protein encoded by the Sl locus, and has also been called “kit ligand” (KL) and mast cell growth factor (MGF), based on the biological properties used to identify it (reviewed in Tsujimura, Pathol Int 1996, 46:933-938; Loveland, et al., J. Endocrinol 1997, 153:337-344; Vliagoftis, et al,, Clin Immunol 1997, 100:435-440; Broudy, Blood 1997, 90: 1345-1364; Pignon, Hermatol Cell Ther 1997, 39: 1 14-1 16; and Lyman, et al., Blood 1998, 91 : 1 101 -1 134.). Herein the abbreviation SCF refers to the physiological ligand for c-kit.
SCF is synthesized as a transmembrane protein with a molecular weight of 220 or 248 Dalton, depending on alternative splicing of the mRNA to encode exon 6. The larger protein can be proteolytically cleaved to form, a soluble, glycosylated protein which noncovalently dimerizcs. Both the soluble and membrane-bound forms of SCF can bind to and activate c-kit. For example, in the skin, SCF is predominantly expressed by fibroblasts, keratinocytes, and endothelial cells, which modulate the activity of melanocytes and mast cells expressing c-kit. In bone, marrow stromal cells express SCF and regulate hematopoiesis of c-kit expressing stem cells. In the gastrointestinal tract, intestinal epithelial cells express SCF and affect the interstitial cells of Cajal and intraepithelial lymphocytes. In the testis, Sertoli cells and granulosa cells express SCF which regulates spermatogenesis by interaction with c-kit on germ cells.
PATENT
PATENT
WO 2008064265
PATENT
WO 2008064255
PATENT
Fragments in the clinic: PLX3397
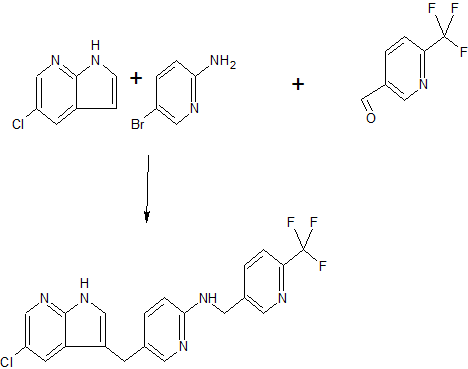
Practical Fragments covers a wide variety of journals. J. Med. Chem., Bioorg. Med. Chem. Lett., Drug Disc. Today, and ACS Med. Chem. Lett. are all well-represented, but we also range further afield, from biggies such asNature and Science to more niche titles such as ChemMedChem, Acta. Cryst. D., and Anal. Chim. Acta. The increasingly clinical relevance of fragment-based approaches is highlighted by a recent paper by William Tap and a large group of collaborators appearing in the New England Journal of Medicine. This reports on the results of the Daiichi Sankyo (née Plexxikon) drug PLX3397 in a phase I trial for tenosynovial giant-cell tumor, a rare but aggressive cancer of the tendon sheath.
The story actually starts with a 2013 paper by Chao Zhang and his Plexxikon colleagues in Proc. Nat. Acad. Sci. USA. The researchers were interested in inhibiting the enzymes CSF1R (or FMS) and KIT; both kinases are implicated in cancer as well as inflammatory diseases. The team started with 7-azaindole, the same fragment they used to discover vemurafenib. Structural studies of an early derivative, PLX070, revealed a hydrogen bond between the ligand oxygen and a conserved backbone amide. Further building led to PLX647, with good activity against both CSF1R and KIT. Selectivity profiling against a panel of 400 kinases revealed only two others with IC50values < 0.3 µM. The molecule was active in cell-based assays, had good pharmacokinetics in mice and rats, and was active in rodent models of inflammatory disease.
The new paper focuses on the results of a clinical trial with PLX3397, a derivative of PLX647. Despite its close structural similarity to PLX647, it binds to CSF1R in a slightly different manner. Both inhibitors bind to the inactive form of the kinase, but PLX3397 also recruits the so-called juxtamembrane domain of the kinase to stabilize this autoinhibited conformation. Pharmacokinetic and pharmacodynamics studies in animals were also positive.

http://practicalfragments.blogspot.in/2015/10/fragments-in-clinic-plx3397.html
Tenosynovial giant-cell tumor seems to be dependent on CSF1R, so the researchers performed a phase 1 dose-escalation study with an extension in which patients treated with the chosen phase 2 dose were treated longer. Of the 23 patients in this extension, 12 had a partial response and 7 had stable disease. A quick search ofclinicaltrials.gov reveals that PLX3397 is currently in multiple trials for several indications, including a phase 3 trial for giant cell tumor of the tendon sheath.
Several lessons can be drawn from these studies. First, as the authors note, one fragment can give rise to multiple different clinical candidates. Indeed, in addition to vemurafenib, 7-azaindole was also the starting point forAZD5363. This is a good counterargument to those who believe that novelty is essential in fragments.
A second, related point is that selectivity is also not necessary for a fragment. The fact that 7-azaindole comes up so frequently as a kinase-binding fragment has not prevented researchers from growing it into remarkably selective inhibitors. An obvious corollary is that even subtle changes to a molecule can have dramatic effects: the added pyridyl nitrogen in PLX3397 is essential for stabilizing a unique conformation of the enzyme.
| Patent ID | Date | Patent Title |
|---|---|---|
| US2015265586 | 2015-09-24 | COMPOUNDS MODULATING C-FMS AND/OR C-KIT ACTIVITY AND USES THEREFOR |
| US2014243365 | 2014-08-28 | COMPOUNDS MODULATING C-FMS AND/OR C-KIT ACTIVITY AND USES THEREFOR |
| US8722702 | 2014-05-13 | Compounds modulating c-fms and/or c-kit activity and uses therefor |
| US2014045840 | 2014-02-13 | COMPOUNDS AND METHODS FOR KINASE MODULATION, AND INDICATIONS THEREFOR |
| US2013274259 | 2013-10-17 | KINASE MODULATION AND INDICATIONS THEREFOR |
| US8404700 | 2013-03-26 | Compounds modulating c-fms and/or c-kit activity and uses therefor |
| US2011230482 | 2011-09-22 | COMPOUNDS MODULATING C-FMS AND/OR C-KIT ACTIVITY |
| US7893075 | 2011-02-22 | Compounds modulating c-fms and/or c-kit activity and uses therefor |
//////1029044-16-3, Pexidartinib , PLX-3397, PHASE3
FC(F)(F)c1ccc(cn1)CNc2ccc(cn2)Cc4cnc3ncc(Cl)cc34