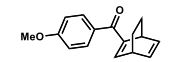
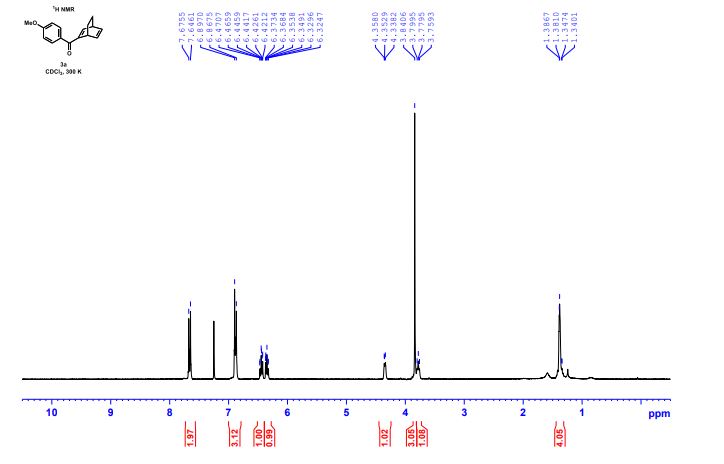
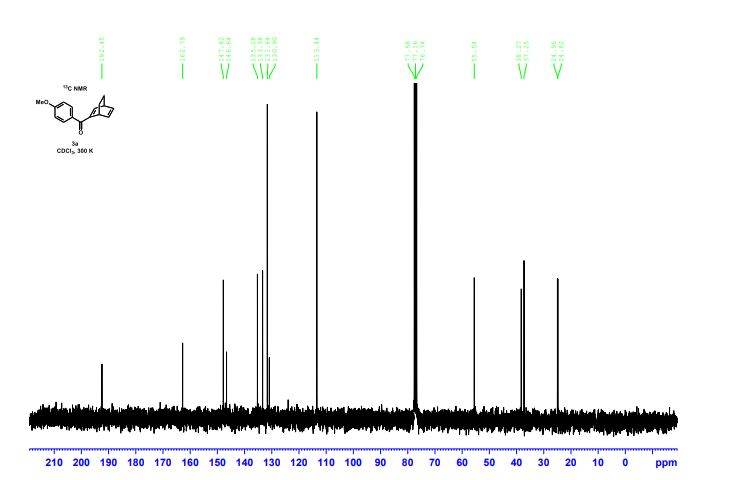
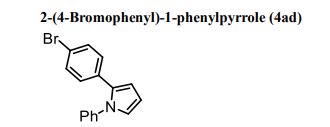
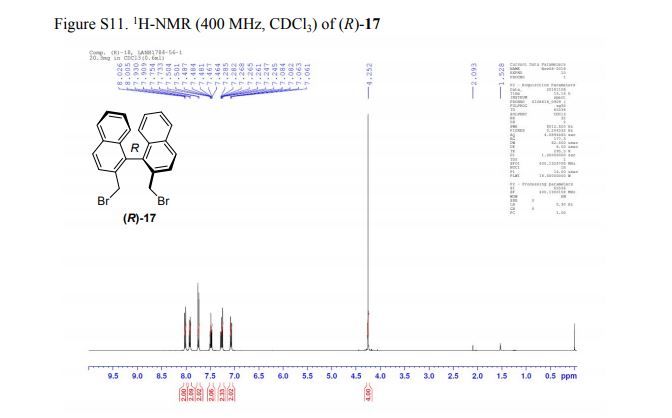
(R)-2,2′-bis(bromomethyl)-1,1′-binaphthalene ((R)-17) was prepared in the identical manner and had identical analytical properties to those given here.
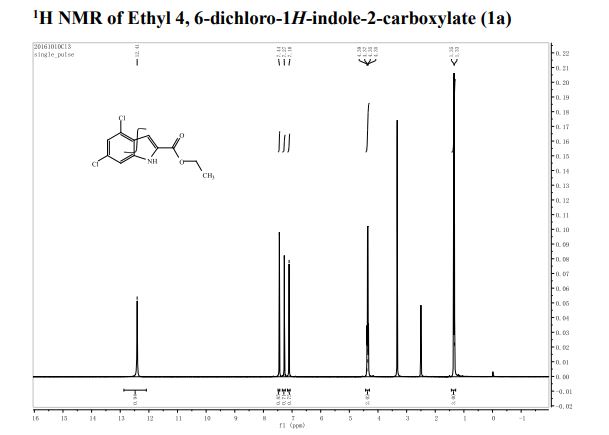
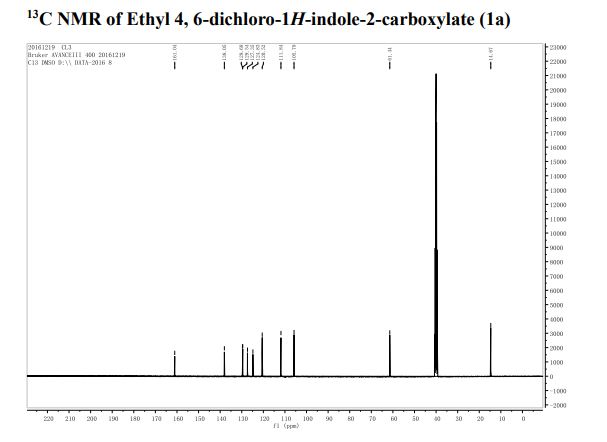
1H NMR (400 MHz, CDCl3): δ 4.25 (4H, s, 2 × CH2), 7.07 (2H, dd, J = 8.4, 0.8 Hz, ArH), 7.27 (2H, ddd, J = 8.4, 6.8, 1.2 Hz, ArH), 7.48 (2H, ddd, J = 8.2, 6.8, 1.2 Hz, ArH), 7.74 (2H, d, J = 8.6 Hz, ArH), 7.92 (2H, d, J = 8.2 Hz, ArH), 8.02 (2H, d, J = 8.6 Hz, ArH).
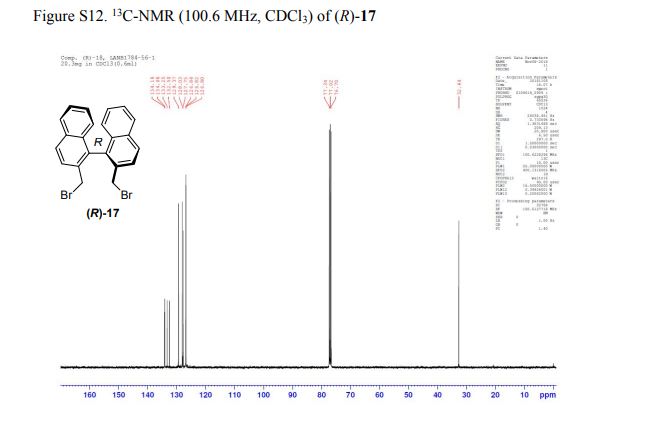
13C NMR (100.6 MHz, CDCl3): δ 32.6 (CH2), 126.80 (ArCH), 126.82 (ArCH), 126.84 (ArCH), 127.7 (ArCH), 128.0 (ArCH), 129.4 (ArCH), 132.5 (quaternary ArC), 133.3 (quaternary ArC), 134.1 (quaternary ArC), 134.2 (quaternary ArC).
[α]20D = +173.8° (c = 1.0, CHCl3).
An advanced process for large scale (500 g) preparation of a (3Z,5Z)-2,7-dihydro-1H-azepine-derived chiral tridentate ligand (Hamari ligand), widely used for asymmetric synthesis of tailor-made α-amino acids via the corresponding glycine Schiff base Ni(II) complex, is disclosed. The process includes amidation, bis-alkylation, and precipitation/purification of the target compound by TFA as a counterion.
Large Scale Synthesis of Chiral (3Z,5Z)-2,7-Dihydro-1H-azepine-Derived Hamari Ligand for General Asymmetric Synthesis of Tailor-Made Amino Acids
//////////////////























 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..