
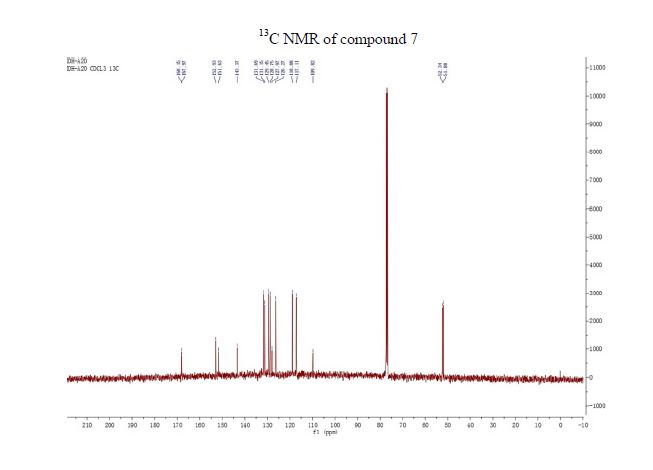
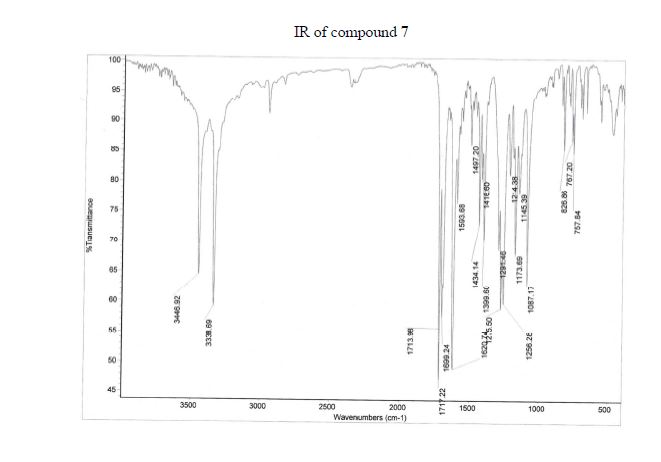
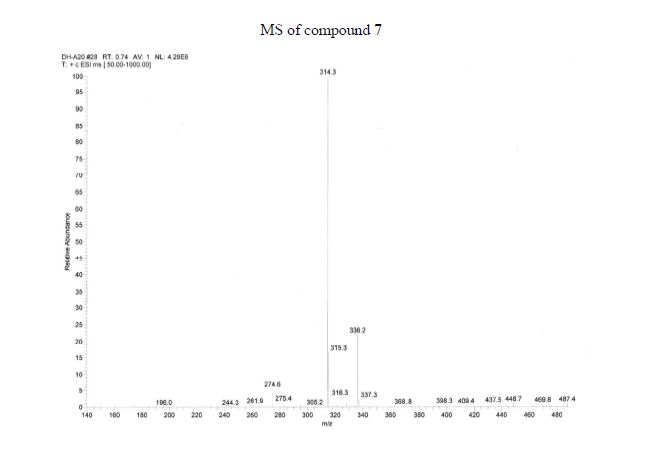
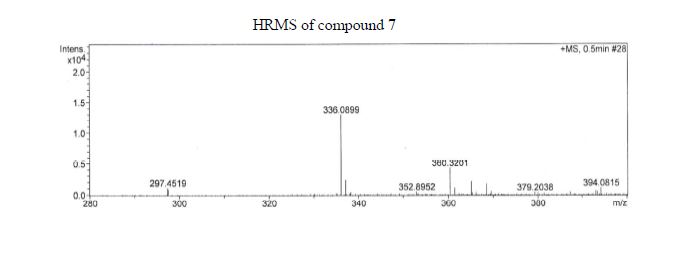
Over the last 20 years organic carbamates have found numerous applications in pesticides, fungicides, herbicides, dyes, pharmaceuticals, cosmetics, and as protecting groups and intermediates for polyurethane synthesis. Recently, in order to avoid phosgene-based synthesis of carbamates, many environmentally benign and alternative pathways have been investigated. However, few examples of carbamoylation of aniline in continuous-flow apparatus have been reported. In this work, we report a high-yielding, dimethyl carbonate (DMC)-mediated carbamoylation of aniline in a fixed-bed continuously fed reactor employing basic zinc carbonate as catalyst. Several variables of the system have been investigated (i.e. molar ratio of reagents , flow rate, and reaction temperature) to optimize the operating conditions of the system.


Highly Selective Phosgene-Free Carbamoylation of Aniline by Dimethyl Carbonate under Continuous-Flow Conditions
PIETRO TUNDO

Profile:
PIETRO R. TUNDO is Professor of Organic Chemistry at Ca’ Foscari University of Venice (Italy).
He was guest researcher and teacher at College Station (Texas,1979-1981), Potsdam (New York, 1989-90) and Syracuse (New York, 1991-92), Chapel Hill, (North Carolina, 1995).
He is Member of the Bureau of IUPAC.
P: Tundo is author of about 300 scientific publications, 40 patents and many books.
His scientific interests are in the field of organic synthesis in selective methylations with low environmental impact, continuous flow chemistry, chemical detoxification of contaminants, hydrodehalogenation under multiphase conditions, phase-transfer catalysis (gas-liquid phase-transfer catalysis, GL-PTC), synthesis of crown-ethers and functionalized cryptands, supramolecular chemistry, heteropolyacids, and finally safe alternatives to harmful chemicals.
He is the sole author of the book “Continuous flow methods in organic synthesis” E. Horwood Pub., Chichester, UK, 1991 (378 pp.), and editor of about 15 books.
P. Tundo was President of Organic and Biomolecular Chemistry Division of IUPAC (biennium 2007-2009) and holder of the Unesco Chair on Green Chemistry (UNTWIN N.o 731). He founded and was Chairman (2004-2016) of the Working Party on “Green and Sustainable Chemistry” of Euchems (European Association for Chemical and Molecular Sciences).
Founder of the IUPAC International Conferences Series on Green Chemistry, he was awarded by American Chemical Society on 1983 (Kendall Award, with Janos Fendler), and by Federchimica (Italian association of chemical industries) on 1997 (An Intelligent Future).
P. Tundo coordinated many institutional and industrial research projects (EU, NATO, Dow, ICI, Roquette) and was Director of the 10 editions of the annual Summer School on Green Chemistry (Venezia, Italy) sponsored by the EU, UNESCO and NATO.
He was guest researcher and teacher at College Station (Texas,1979-1981), Potsdam (New York, 1989-90) and Syracuse (New York, 1991-92), Chapel Hill, (North Carolina, 1995).
He is holder of the Unesco Chair on Green Chemistry (UNTWIN N.o 731) and author of about 260 scientific publications and 30 patents.
Scientific interests are in the field of organic synthesis in selective methylations with low environmental impact, continuous flow chemistry, chemical detoxification of contaminants, hydrodehalogenation under multiphase conditions, phase-transfer catalysis (gas-liquid phase-transfer catalysis, GL-PTC), synthesis of crown-ethers and functionalized cryptands, supramolecular chemistry and finally, heteropolyacids.
He is the sole author of the book “Continuous flow methods in organic synthesis” E. Horwood Pub., Chichester, UK, 1991 (378 pp.), and editor of about 15 books.
P. Tundo was President of Organic and Biomolecular Chemistry Division of IUPAC (biennium 2007-2009) and presently is Chairman of Working Party of “Green and Sustainable Chemistry” of Euchems (European Association for Chemical and Molecular Sciences).
Founder of the IUPAC International Conferences Series on Green Chemistry, he was awarded by American Chemical Society on 1983 (Kendall Award, with Janos Fendler), and by Federchimica (Italian association of chemical industries) on 1997 (An Intelligent Future).
P. Tundo co-ordinated many institutional and industrial research projects (EU, NATO, Dow, ICI, Roquette) and was Director of the 10 editions of the annual Summer School on Green Chemistry (Venezia), the latter sponsored by the EU, UNESCO and NATO.
Contact:
Professor of Organic Chemistry
Ca’ Foscari University of Venice
IUPAC Bureau Member
Tel. +39 041 2348642
Mob. +39 349 3486191
E-mail: tundop@unive.it
| Phone | 041 234 8642 / Lab .: 041 234 8669 |
|---|---|
| tundop@unive.it green.chemistry@unive.it – 6th IUPAC Conference on Green Chemistry unescochair@unive.it – TUNDO Pietro |
|
| Fax | 041 234 8620 |
| Web | www.unive.it/persone/tundop |
////////Carbamoylation of Aniline, Dimethyl Carbonate, Continuous-Flow Conditions, flow synthesis
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This article is a compilation for educational purposes only.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent













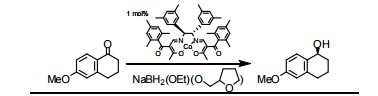
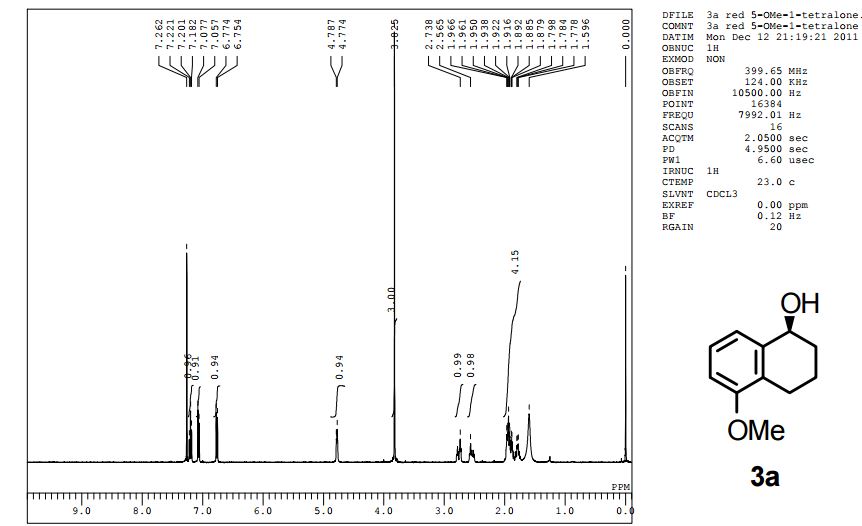
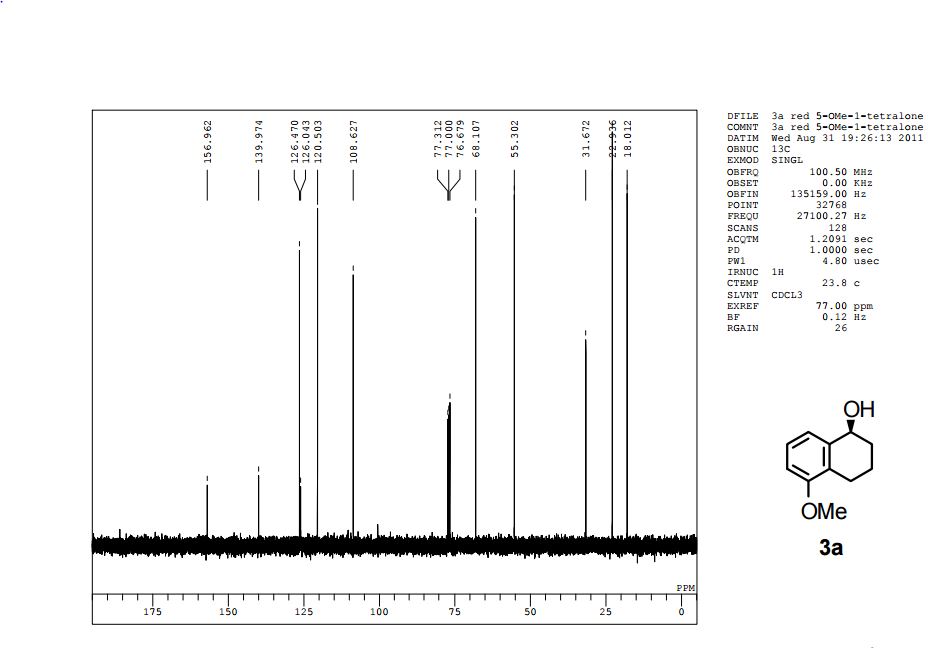



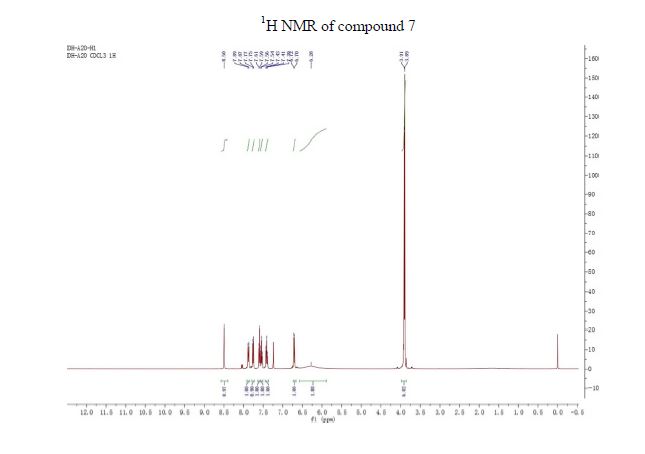



 The presentation will load below
The presentation will load below CHEMTRIX
CHEMTRIX