
Specific Stereoisomeric Conformations Determine the Drug Potency of Cladosporin Scaffold against Malarial Parasite
https://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.8b00565
Pronay Das, Palak Babbar, Nipun Malhotra, Manmohan Sharma, Gorakhnath R Jachak, Rajesh G. Gonnade, Dhanasekaran Shanmugam, Karl Harlos, Manickam Yogavel, amit sharma, and D. Srinivasa Reddy
Pronay Das†ab, Palak Babbar†c, Nipun Malhotra†c, Manmohan Sharmac , Goraknath R. Jachakab , Rajesh G. Gonnadebd, Dhanasekaran Shanmugambe, Karl Harlosf , Manickam Yogavelc , Amit Sharmac *, and D. Srinivasa Reddyab* †All three have contributed equally to this work.
aOrganic Chemistry Division, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune 411008, India
b Academy of Scientific and Innovative Research (AcSIR), New Delhi 110025, India
cMolecular Medicine Group, International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi 110067, India dCenter for Material Characterization, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune 411008, India
e Biochemical Sciences Division, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune 411008, India
fDivision of Structural Biology, Welcome Trust Centre for Human Genetics, The Nuffield Department of Medicine, University of Oxford, Oxford OX3 7BN, UK
J. Med. Chem., Just Accepted Manuscript
DOI: 10.1021/acs.jmedchem.8b00565
Publication Date (Web): May 21, 2018
Copyright © 2018 American Chemical Society
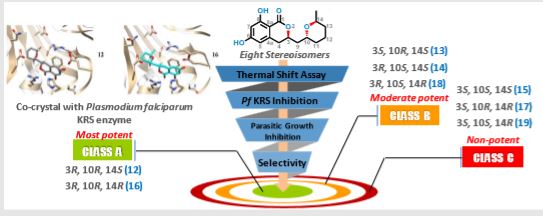
The dependence of drug potency on diastereomeric configurations is a key facet. Using a novel general divergent synthetic route for a three-chiral centre anti-malarial natural product cladosporin, we built its complete library of stereoisomers (cladologs) and assessed their inhibitory potential using parasite-, enzyme- and structure-based assays.
We show that potency is manifest via tetrahyropyran ring conformations that are housed in the ribose binding pocket of parasite lysyl tRNA synthetase (KRS). Strikingly, drug potency between top and worst enantiomers varied 500-fold, and structures of KRS-cladolog complexes reveal that alterations at C3 and C10 are detrimental to drug potency where changes at C3 are sensed by rotameric flipping of Glutamate332.
Given that scores of anti-malarial and anti-infective drugs contain chiral centers, this work provides a new foundation for focusing on inhibitor stereochemistry as a facet of anti-microbial drug development.
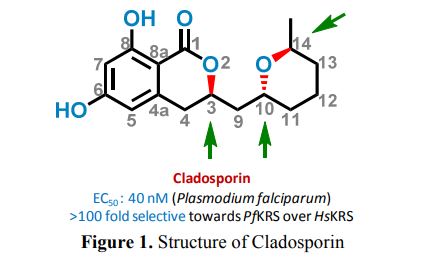
Cladosporin (12) displays exquisite selectivity for the parasite lysyl-tRNA synthetase over human enzyme. This species specific selectivity of cladosporin has been previously described through comprehensive sequence alignment, where the residues val329 and ser346 seem to be sterically crucial for accommodating the methyl moiety of THP ring10. The structural features of compound 12 clearly indicate the presence of three stereocenters, and therefore 2n (n=3) i.e., eight stereoisomers are possible (Fig.1). Till date, only one asymmetric total synthesis of cladosporin13 has been achieved which was followed by another report of formal syntheses14. Here, we have developed a general chemical synthesis route to synthetically access all the eight possible stereoisomers of compound 12.
cladosporin (compound 12) (0.052 g) as a white solid with a yield of 54 %. Melting point: 171-173 °C; [α]25 D = -15.75 (c = 0.6, EtOH); IR υmax(film): cm-1 3416, 3022, 1656, 1218; 1H NMR (400 MHz, CDCl3): δ 11.06 (s, 1H), 7.47 (br. s., 1H), 6.29 (s, 1H), 6.16 (s, 1H), 4.68 (t, J = 9.8 Hz, 1H), 4.12 (s, 1H), 4.01 (s, 1H), 2.89 – 2.75 (m, 2H), 2.00 – 1.94 (m, 1H), 1.87 – 1.81 (m, 1H), 1.70 – 1.63 (m, 4H), 1.35 (d, J = 6.1 Hz, 2H), 1.23 (d, J = 6.7 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 169.9, 164.3, 163.1, 141.8, 106.7, 102.0, 101.5, 76.3, 68.0, 66.6, 39.3, 33.6, 30.9, 18.9, 18.1; HRMS calculated for C16H21O5 [M + H]+ 293.1384, observed 293.1379.

The research interests of his group lie in issues related to application of oriented organic synthesis, in particular total synthesis of biologically active natural products, medicinal chemistry and crop protection. This team has been credited with having accomplished total synthesis of more than 25 natural products with impressive biological activities. “Some of our recent achievements include identification of potential leads, like antibiotic compound based on hunanamycin natural product for treating food infections, anti-diabetic molecule in collaboration with an industry partner and anti-TB compound using a strategy called ‘re-purposing of a drug scaffold’,” said Reddy.
A total of two awardees out of four were from CSIR institutes. In addition to Reddy, Rajan Shankarnarayanan, CSIR – CCMB, Hyderabad (basic sciences), also was conferred with the award. Vikram Mathews, CMC, Vellore (medical research) and Prof Ashish Suri, AIIMS, New Delhi (clinical research), were the others to receive the awards.
With more than 80 scientific publications and 35 patents, Reddy is one of the most prominent scientists in the city and has already been honoured with the Shanti Swarup Bhatnagar prize in chemical sciences. Reddy is also a nominated member of the scientific body of Indian Pharmacopoeia, government of India and was elected as a fellow of the Telangana and Maharashtra Academies of Sciences in addition to the National Academy of Sciences, India (NASI).
//////////CLADOSPORIN, NCL, CSIR, SRINIVASA REDDY, PUNE, MALARIA

















Sorry, the comment form is closed at this time.