Feb 012017
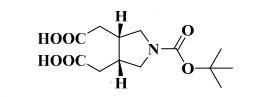
2,2′-(1-(tert-Butoxycarbonyl)pyrrolidine-3,4-diyl)diacetic Acid
2,2′-(1-(tert-Butoxycarbonyl)pyrrolidine-3,4-diyl)diacetic Acid
as a white solid. Mp: 162–163 °C, % purity: 94.09% (HPLC);
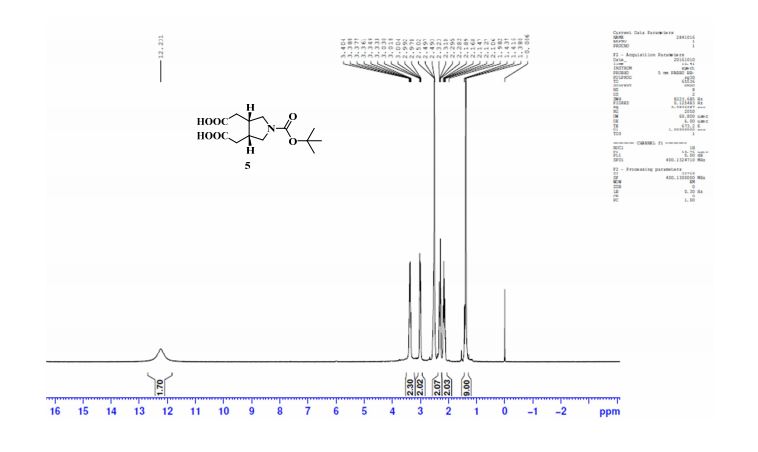
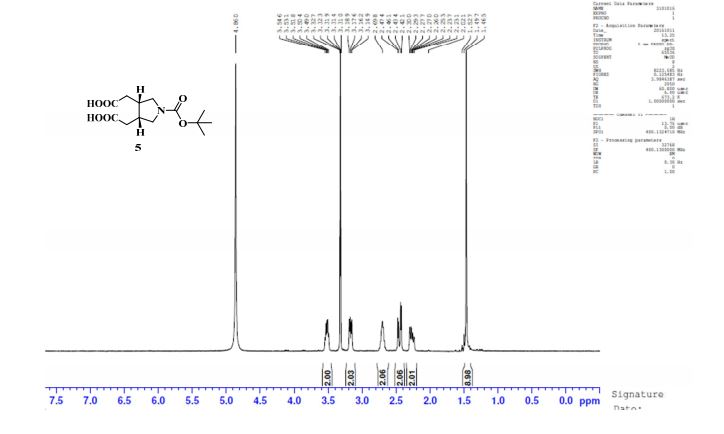
1H NMR (DMSO-d6, 400 MHz) δ: 1.38 (s, 9H), 2.10–2.18 (m, 2H), 2.28–2.32 (m, 2H), 2.49–2.50 (m, 2H, merged with DMSO peak), 2.97–3.03 (m, 2H), 3.33–3.40 (m, 2H), 12.23 (bs, 2H); 1H NMR (CD3OD, 400 MHz) δ: 1.46 (s, 9H), 2.26 (ddd, J1 = 2.8 Hz, J2 = 9.2 Hz, J3 = 16.0 Hz, 2H), 2.43 (dd, J1 = 5.2 Hz, J2 = 16.0 Hz, 2H), 2.69 (m, 2H), 3.16 (dd, J1 = 5.2 Hz, J2 = 10.8 Hz, 2H), 3.49–3.54 (m, 2H);
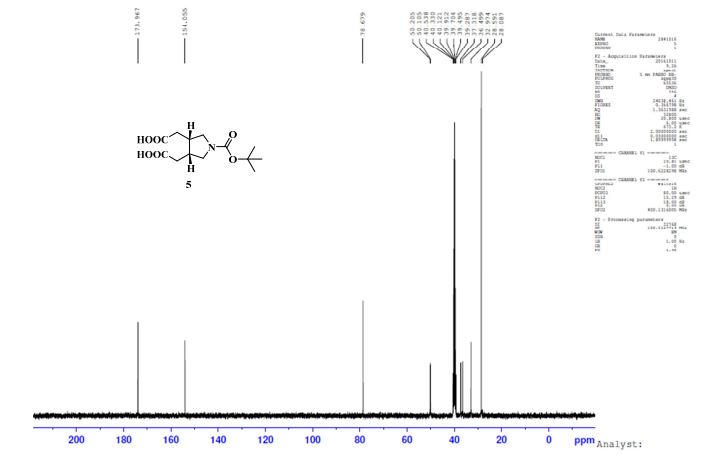
13C NMR (DMSO-d6, 100 MHz) δ: 28.49, 32.97, 36.49, 37.31, 50.10, 50.20, 78.67, 154.05, 173.96;
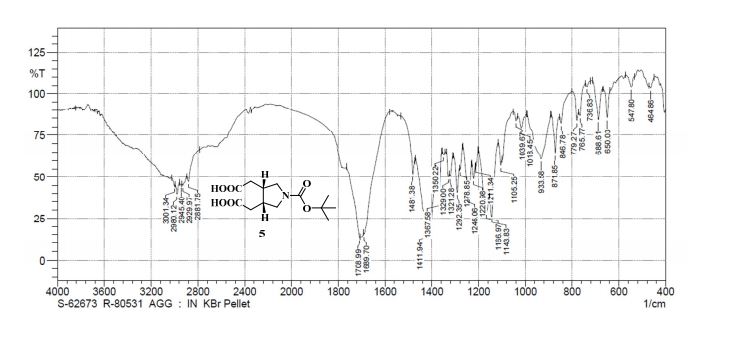
IR (KBr): ν = 871, 933, 1143, 1166, 1292, 1411, 1689, 1708, 2881, 2929, 2980, 3001 cm–1;
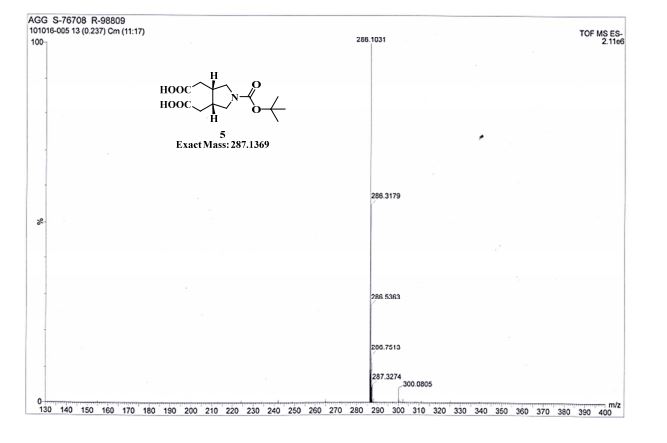
TOFMS: [C13H21NO6 – H+]: calculated 286.1296, found 286.1031(100%).
HPLC conditions were as follows for compound ; Agilent 1100 series, column: YMC J’SPHERE C18 (150 mm X 4.6 mm) 4µm with mobile phases A (0.05% TFA in water) and B (acetonitrile). Detection was at 210 nm, flow was set at 1.0 mL/min, and the temperature was 30 °C (Run time: 45 min). Gradient: 0 min, A = 90%, B = 10%; 5.0 min, A = 90%, B = 10%; 25 min, A = 0%, B = 100%; 30 min, A = 0%, B = 100%, 35 min, A = 90%, B = 10%; 45 min, A = 90%, B = 10%.
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00399
/////////













Sorry, the comment form is closed at this time.