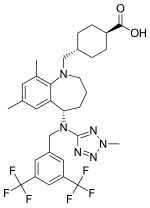
Evacetrapib
The design, development, and scale up of a continuous iridium-catalyzed homogeneous high pressure reductive amination reaction to produce 6, the penultimate intermediate in Lilly’s CETP inhibitor evacetrapib, is described. The scope of this report involves initial batch chemistry screening at milligram scale through the development process leading to full-scale production in manufacturing under GMP conditions. Key aspects in this process include a description of drivers for developing a continuous process over existing well-defined batch approaches, manufacturing setup, and approaches toward key quality and regulatory questions such as batch definition, the use of process analytics, start up and shutdown waste, “in control” versus “at steady state”, lot genealogy and deviation boundaries, fluctuations, and diverting. The fully developed continuous reaction operated for 24 days during a primary stability campaign and produced over 2 MT of the penultimate intermediate in 95% yield after batch workup, crystallization, and isolation.
Development and Manufacturing GMP Scale-Up of a Continuous Ir-Catalyzed Homogeneous Reductive Amination Reaction
ACS Editors’ Choice – This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes.
/////////continuous manufacturing, deviation boundaries, Evacetrapib, flow chemistry, GMP, homogeneous catalysis, hydrogen, hydrogenation, iridium, lot genealogy, LY2484595, online HPLC, process analytical technologies, reductive amination, start up and shutdown transitions, state of control, steady state, [Ir(COD)Cl]2















Sorry, the comment form is closed at this time.