

Lovastatin (Merck’s Mevacor) is a statin drug, used for lowering cholesterol (hypolipidemic agent) in those withhypercholesterolemia to reduce risk of cardiovascular disease. Lovastatin is a naturally occurring compound found in food such asoyster mushrooms,[2] red yeast rice,[3] and Pu-erh.[4]
Medical uses
The primary uses of lovastatin is for the treatment of dyslipidemia and the prevention of cardiovascular disease.[5] It is recommended to be used only after other measures, such as diet, exercise, and weight reduction, have not improved cholesterol levels.[5]
Pleurotus ostreatus, the oyster mushroom, naturally contains up to 2.8% lovastatin on a dry weight basis.[15]
Structure


History
Compactin and lovastatin, natural products with a powerful inhibitory effect on HMG-CoA reductase, were discovered in the 1970s, and taken into clinical development as potential drugs for lowering LDL cholesterol.
However, in 1980, trials with compactin were suspended for undisclosed reasons (rumoured to be related to serious animal toxicity). Because of the close structural similarity between compactin and lovastatin, clinical studies with lovastatin were also suspended, and additional animal safety studies initiated.
In 1982 some small-scale clinical investigations of lovastatin, a polyketide derived natural product isolated from Aspergillus terrus, in very high-risk patients were undertaken, in which dramatic reductions in LDL cholesterol were observed, with very few adverse effects. After the additional animal safety studies with lovastatin revealed no toxicity of the type thought to be associated with compactin, clinical studies resumed.
Large-scale trials confirmed the effectiveness of lovastatin. Observed tolerability continued to be excellent, and lovastatin was approved by the US FDA in 1987.
Lovastatin at its maximal recommended dose of 80 mg daily produced a mean reduction in LDL cholesterol of 40%, a far greater reduction than could be obtained with any of the treatments available at the time. Equally important, the drug produced very few adverse effects, was easy for patients to take, and so was rapidly accepted by prescribers and patients. The only important adverse effect is myopathy/rhabdomyolysis. This is rare and occurs with all HMG-CoA reductase inhibitors.
Mechanism of action
Lovastatin is an inhibitor of 3-hydroxy-3methylglutaryl-coenzyme A reductase (HMG-CoA reductase), an enzyme which catalyzes the conversion of HMG-CoA to mevalonate. Mevalonate is a required building block for cholesterol biosynthesis and lovastatin interferes with its production by acting as a competitive inhibitor for HMG-CoA which binds to the HMG-CoA reductase. Lovastatin, being inactive in the native form, the form in which it is administered, is hydrolysed to the β-hydroxy acid form in the body and it is this form which is active. Presumably, the reductase acts on the hydrolyzed lovastatin to reduce the carboxylic acid moiety.
Discovery, Biochemistry and Biology
It is now generally accepted that a major risk factor for the development of coronary heart disease is an elevated concentration of plasma cholesterol, especially lowdensity lipoprotein (LDL) cholesterol. The objective is to decrease excess levels of cholesterol to an amount consistent with maintainence of normal body function. Cholesterol is biosynthesized in a series of more than 25 separate enzymatic reactions that initially involves 3 successive condensations of acetyl-CoA units to form a 6-carbon compound, 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA). This is reduced to mevalonate and then converted in a series of reactions to the isoprenes that are building blocks of squalene, the immediate precursor to sterols, which cyclizes to lanosterol (a methylated sterol) and further metabolized to cholesterol. A number of early attempts to block the synthesis of cholesterol resulted in agents that inhibited late in the biosynthetic pathway between lanosterol and cholesterol. A major rate limiting step in the pathway is at the level of the microsomal enzyme which catalyzes the conversion of HMG CoA to mevalonic acd and which has been considered to be a prime target for pharmacologic intervention for several years.
HMG CoA reductase occurs early in the biosynthetic pathway and is among the first commited steps to cholesterol formulation. Inhibition of this enzyme could lead to accumulation of HMG CoA, a water-soluble intermediate that is then capable of being readily metabolized that is then capable of being readily metabolized to simpler molecules. This inhibition of reductase would nto lead to accumulation of lipophylic intermediates having a formal sterol ring.
Lovastatin is the first specific inhibitor of HMG CoA reductase to receive approval for the treatment of hypercholesterolemia. The first breakthrough in efforts to find a potent, specific, competitive inhibitor of HMG CoA reductase occurred in 1976 when Endo et al reported discovery of mevastatin, a highly functionalized fungal metabolite, isolated from cultures of Penicillium citrium. Mevastatin was demonstrated to be an unusually potent inhibitor of the target enzyme and of cholesterol biosynthesis. Subsequent to the first reports describing mevastatin, efforts were initiated to search for other naturally occurring inhibitors oh HMG CoA reductase. This led to the discovery of a novel fungal metabolite – Lovastatin. The structure of Lovastatin was determined to be different from that of mevastatin by the presence of a 6 alphamethyl group in the hexahydronaphthalene ring.
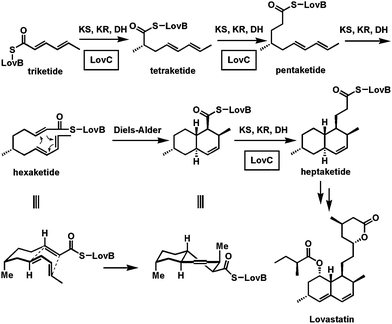
Key points from the study of the Biosynthesis of Lovastatin :-
– Lovastatin is comprised of 2 polyketide chains derived from acetate that are 8- and 4-
carbons long coupled in head to tail fashion.
– 6 alphamethyl group and the methyl group on the 4-carbon side chain are derived from
the methyl group of methionine, and
– 6 alphamethyl group is added before closure of the rings.
This implies that lovastatin is a unique compound synthesized by A. terreus and that mevastatin is not an intermediate in its fornmation.
Cholesterol Biosynthetic Pathway

The HMG CoA reductase reaction

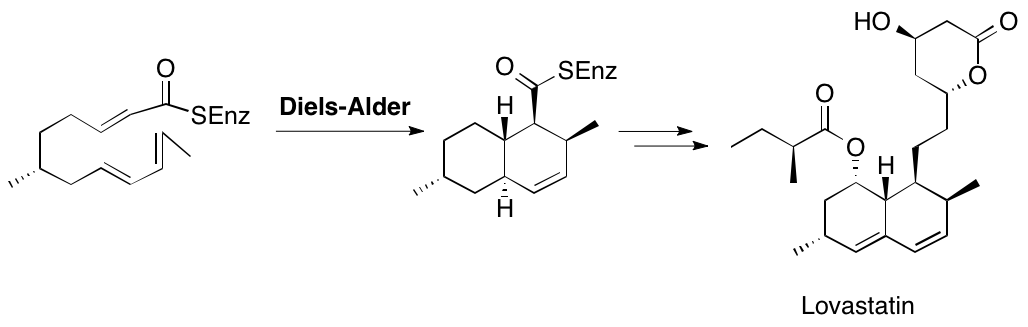
Biosynthesis — Diels-Alder Catalyzed Cyclization
In vitro formation of a triketide lactone using a genetically-modified protein derived from 6-deoxyerythronolide B synthase has been demonstrated. The stereochemistry of the molecule supports the intriguing idea that an enzyme-catalyzed Diels-Alder reaction may occur during assembly of the polyketide chain. It thus appears that biological Diels-Alder reactions may be triggered by generation of reactive triene systems on an enzyme surface.


Biosynthesis – Using Broadly specific Acyltransferase
It has been found that a dedicated acyltransferase, LovD, is encoded in the lovastatin biosynthetic pathway. LovD has a broad substrate specificity towards the acyl carrier, the acyl substrate and the decalin acyl acceptor. It efficiently catalyzes the acyl transfer from coenzyme A thoesters or N-acetylcysteamine (SNAC) thioesters to monacolin J.
The biosynthesis of Lovastatin is coordinated by two iterative type I polyketide synthases and numerous accessory enzymes. Nonketide, the intermediate biosynthetic precursor of Lovastatin, is assembeled by the upstream megasynthase LovB (also known as lovastatin nonaketide synthase), enoylreductase LovC, and CYP450 oxygenases. The five carbon unit side chain is synthesized by LovF (also known as lovastatin diketide synthase) through a single condensation diketide undergoes methylation and reductive tailoring by the individual LovF catalytic domains to yield an α-S-methylbutyryl thioester covalently attached to the phosphopantetheine arm on the acyl carrier protein (ACP) domain of LovF. Encoded in the gene cluster is a 46kDa protein, LovD, which was initially identified as an esterase homolog. LovD, which was initially identified as an esterase homolog. LovD was suggested to catalyze the last step of lovastatin biosynthesis that regioselectively transacylates the acyl group from LovF to the C8 hydroxyl group of the Nonaketide to yield Lovastatin.




K. Auclair, A. Sutherland, J. Kennedy, D. J. Witter, J. P. Van den Heever, C. R. Hutchinson and J. C. Vederas, Lovastatin Nonaketide Synthase Catalyses An Intramolecular Diels-Alder Reaction Of A Substrate Analogue, J. Am. Chem. Soc., 2000, 122, 11519-11520. DOI: 10.1021/ja003216+
http://pubs.rsc.org/en/content/articlelanding/2013/np/c2np20069d/unauth#!divAbstract

http://www.hindawi.com/journals/bmri/2012/196264/#B30
- Z. Jia, X. Zhang, Y. Zhao, and X. Cao, “Enhancement of lovastatin production by supplementing polyketide antibiotics to the submerged culture of Aspergillus terreus,” Applied Biochemistry and Biotechnology, vol. 160, no. 7, pp. 2014–2025, 2010.

Patent
https://www.google.com/patents/US6307066

PATENT
https://www.google.com/patents/WO2002009697A1?cl=en



https://www.google.com/patents/EP0625208B1?cl=en
Total Synthesis
A major bulk of work in the synthesis of Lovastatin was done by M. Hirama in the 1980’s. Hirama synthesized Compactin and used one of the intermediates to follow a different path to get to Lovastatin. The synthetic sequence is shown in the schemes below. The γ-lactone was synthesized using Yamada methodology starting with aspartic acid. Lactone opening was done using lithium methoxide in methanol and then silylation to give a separable mixture of the starting lactone and the silyl ether. The silyl ether on hydrogenolysis followed by Collins oxidation gave the aldehyde. Stereoselective preparation of (E,E)-diene was accomplished by addition of trans-crotyl phenyl sulfone anion, followed by quenching with Ac2O and subsequent reductive elimination of sulfone acetate. Condensation of this with Lithium anion of dimethyl methylphosphonate gave compound 1.Compound 2 was synthesized as shown in the scheme in the synthetic procedure. Compounds 1 and 2 were then combined together using 1.3eq sodium hydride in THF followed by reflux in chlorobenzene for 82 hrs under nitrogen to get the enone 3.
Simple organic reactions were used to get to Lovastatin as shown in the scheme.


Pharmacopoeia Information
Lovastatin tablets are preserved in well closed, light resistant containers. Protected from light and stored either in a cool place or at controlled room temperature.
Lovastatin tablets are tested for Dissolution and Assay as per the USP.
Limit for Dissolution – Not less than 80% (Q) of the labeled amount of Lovastatin is dissolved in 30 mins.
Limit for Assay – Each tablet contains not less than 90% and not more than 110% of the labeled amount of Lovastatin, tested by HPLC analysis.
Lovastatin raw material contains 5 impurities – A, B, C, D and E (as shown below).

Market brands and other analogues
There are other derivatives of Lovastatin which possess cholesterol reducing activity. Simvastatin (Zocor®) is another statin closely related to Lovastatin, differing only by the presence of a methyl group in the butanoyl ester moiety. Both effective in lowering total cholesterol.
Another statin having vastly different structure but a popular drug – Atorvastatin (Lipitor®), administered as a calcium salt is a pyrrole derivative and a synthetic compound rather than a natural product.
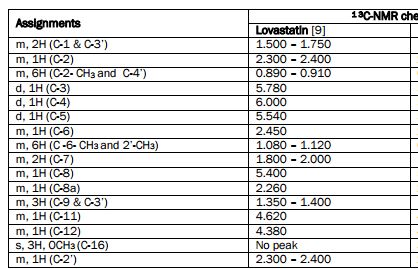
NMR

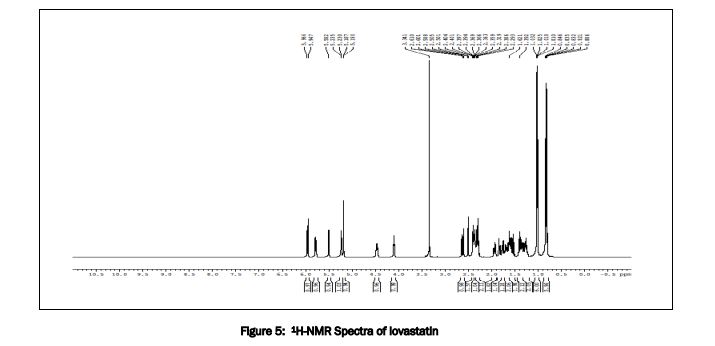
1 H NMR spectrum of lovastatin, 300 MHz, solvent CDCl 3 .
 UV LOVASTATIN
UV LOVASTATIN
Figure 6. The mean FT-IR spectra (the calibration set) and variables selected after application of UVE-PLS for modelling lovastatin (triangles) and wavenumbers for characteristic peaks for lovastatin IR spectrum (dots).
PATENT
https://www.google.com/patents/EP0702679B1?cl=en
Lovastatin is produced as a secondary metabolite of the fungusAspergillus terreus (US 4,231,938) deposited in American Type Culture Collection under Nos. ATCC 20541, ATCC 20542, and Monascus ruberdeposited in Fermentation Research Institute Agency of Industrial Science and Technology, Ministry of International Trade and Industry, Japan (DE 30 06 216 A1) under No. Ferm 4822. Other kinds of microorganisms producing lovastatin are known as well, e.g. a mutant of the microorganism Aspergillus terreus andAspergillus oryzae marked ATCC 74135.
Lovastatin is chemically 1′,2′,6′,7′,8a’-hexahydro-3,5-dihydroxy-2′,6′-dimethyl-8′-2″-methyl-1″-oxobutoxy)-1-naphtalene heptanoic acid-5-lactone (Stubbs et al., 1986) of the formula (EP 0 033 537 A1)
An active form of lovastatin is also an acid, which is chemically 1,2,6,8,8a-hexahydro-β,δ-dihydroxy-1-naphtalene heptanoic acid (Alberts et al., 1980) of the formula (EP 0 022 478 A1)
The lactone form of lovastatin is used as an agent for reducing cholesterol level in blood (Scott M.G. and Vega G.L, 1985). It inhibits the biosynthesis of mevalonic acid by inhibition of 3-hydroxy-3-methylglutaryl A reductase coenzyme (HMG-CoA reductase, E.C. 1.1.1.34) (Zubay et al., 1984).
Prior Art
After the completed fermentation, lovastatin is present in the broth in the lactone form (compound I) and in the acid form (compound II). In the isolation process as disclosed in EP 0 033 536 A2, lovastatin is extracted from the broth with ethyl acetate. The extract is concentrated by vacuum distillation. Since lovastatin is present in the lactone form as well as in the acid form and only the lactone is of commercial interest, the acid form should be converted into the lactone. The lactonisation is carried out by the reflux of the concentrate in toluene at 106 °C for 2 hours. After the lactonisation is complete, the solution is concentrated to a small volume. A pure substance is obtained by means of purifying the concentrate on columns packed with silica gel, in the presence of solvents such as ethyl acetate or n-hexane. The collected fractions are again concentrated in vacuo and then pure lovastatin crystallizes in the lactone form.
Due to the sophisticated multi-step procedure and vigorous conditions applied during the isolation, the yields of lovastatin are generally low. Different solvents, which in part exhibit toxicity, are used such as benzene, toluene, acetonitrile or ethyl acetate. Hence working with these solvents endangers the health of the persons involved and poses a problem with respect to the environment.




https://www.google.com/patents/EP0702679B1?cl=en
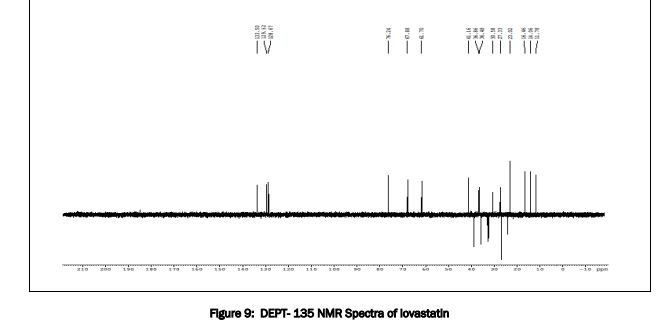
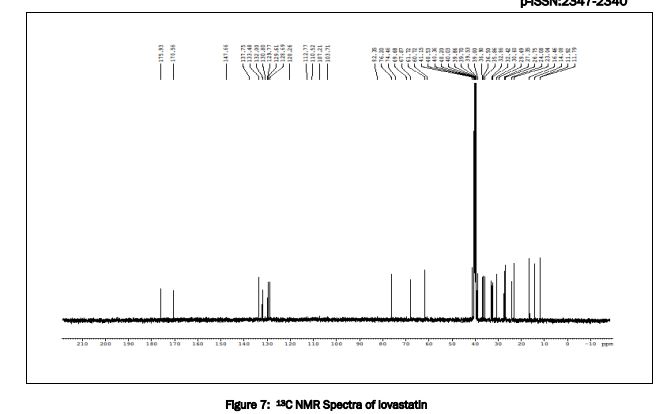
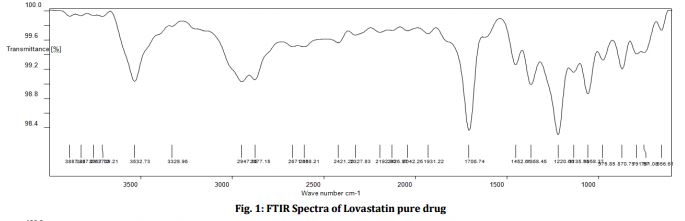
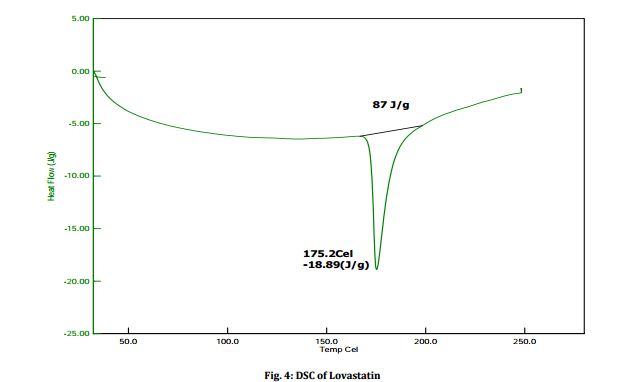
The structure was confirmed by IR spectroscopy (Fig.1), mass spectroscopy (Fig. 2), NMR (Fig. 3) and UV spectroscopy (Fig. 4).
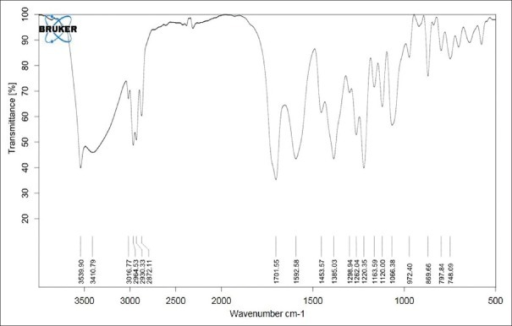 IR spectrum of lovastatin.
IR spectrum of lovastatin.
References
- Neuvonen, PJ; Backman, JT; Niemi, M (2008). “Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin.”. Clinical Pharmacokinetics. 47 (7): 463–74. doi:10.2165/00003088-200847070-00003. PMID 18563955.
- . Gunde-Cimerman N; Cimerman A. (Mar 1995). “Pleurotus fruiting bodies contain the inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase-lovastatin.”. Exp Mycol. 19 (1): 1–6.doi:10.1006/emyc.1995.1001. PMID 7614366.
- Jump up^ Liu J, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fønnebø V (2006).“Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials”.Chin Med. 1 (1): 4. doi:10.1186/1749-8546-1-4. PMC 1761143
 .PMID 17302963.
.PMID 17302963. - Jump up^ Zhao ZJ, Pan YZ, Liu QJ, Li XH (2013). “Exposure assessment of lovastatin in Pu-erh tea”. International Journal of Food Microbiology.164 (1): 26–31. doi:10.1016/j.ijfoodmicro.2013.03.018.PMID 23587710.
- ^ Jump up to:a b “Lovastatin”. The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ^ Jump up to:a b “Mevacor, Altoprev (lovastatin) dosing, indications, interactions, adverse effects, and more”. Medscape Reference. WebMD. Retrieved 17 March 2014.
- ^ Jump up to:a b “Lovastatin”. MedlinePlus. U.S. National Library of Medicine. 15 June 2012. Retrieved 1 December 2012.
- Jump up^ “Lovastatin”. LactMed. U.S. National Library of Medicine. Retrieved 1 December 2012.
- Jump up^ Stöppler, Melissa. “Mevacor Side Effects Center”. RxList. Retrieved 1 December 2012.
- Jump up^ David G. Bailey, J. Malcolm, O. Arnold, J. David Spence (1998). “Grapefruit juice-drug interactions”. Br J Clin Pharmacol 46 (2): 101–110. doi:10.1046/j.1365-2125.1998.00764.x. PMC 1873672. PMID 9723817.
- Jump up^ Kantola T, Kivistö KT, Neuvonen PJ (Apr 1998). “Grapefruit juice greatly increases serum concentrations of lovastatin and lovastatin acid”. Clin Pharmacol Ther. 63 (4): 397–402. doi:10.1016/S0009-9236(98)90034-0. PMID 9585793.
- ^ Jump up to:a b Alberts AW (1998). “Discovery, biochemistry and biology of lovastatin”. The American Journal of Cardiology. 62 (15): 10J–15J.doi:10.1016/0002-9149(88)90002-1. PMID 3055919.
- Jump up^ Katz MS (2005). “Therapy insight: Potential of statins for cancer chemoprevention and therapy”. Nature Clinical Practice Oncology. 2(2): 82–9. doi:10.1038/ncponc0097. PMID 16264880.
- Jump up^ Rao S, Porter DC, Chen X, Herliczek T, Lowe M, Keyomarsi K (July 1999). “Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase”. Proc. Natl. Acad. Sci. U.S.A. 96 (14): 7797–802.doi:10.1073/pnas.96.14.7797. PMC 22141
 . PMID 10393901.
. PMID 10393901. - Jump up^ Alarcón J, Aguila S, Arancibia-Avila P, Fuentes O, Zamorano-Ponce E, Hernández M (Jan–Feb 2003). “Production and purification of statins from Pleurotus ostreatus (Basidiomycetes) strains”. Z Naturforsch C. 58 (1–2): 62–4. doi:10.1515/znc-2003-1-211.PMID 12622228.
- Jump up^ Vederas JC, Moore RN, Bigam G, Chan KJ (1985). “Biosynthesis of the hypocholesterolemic agent mevinolin by Aspergillus terreus. Determination of the origin of carbon, hydrogen and oxygen by 13C NMR and mass spectrometry”. J Am Chem Soc. 107 (12): 3694–701.doi:10.1021/ja00298a046.
- Jump up^ Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, Patchett A, Monaghan R, Currie S, Stapley E, Albers-Schonberg G, Hensens O, Hirshfield J, Hoogsteen K, Liesch J, Springer J (July 1980). “Mevinolin: a highly potent competitive inhibitor of hydroxymethlglutaryl-coenzyme A reductase and a cholesterol-lowering agent”. Proc Natl Acad Sci U S A. 77(7): 3957–61. doi:10.1073/pnas.77.7.3957. PMC 349746
 .PMID 6933445.
.PMID 6933445. - Jump up^ FDA Orange Book Detail for application N019643 showing approval for 20 mg tablets on Aug 31, 1987 and 40 mg tablets on Dec 14, 1988
- Jump up^ Endo, Akira (Oct 2004). “The origin of the statins”. Atheroscler. Suppl. 5 (3): 125–30.doi:10.1016/j.atherosclerosissup.2004.08.033. PMID 15531285.
- ^ Jump up to:a b Bobek P, Ozdín L, Galbavý S (1998). “Dose- and time-dependent hypocholesterolemic effect of oyster mushroom (Pleurotus ostreatus) in rats”. Nutrition. 14 (3): 282–6. doi:10.1016/S0899-9007(97)00471-1. PMID 9583372.
- Jump up^ Hossain S, Hashimoto M, Choudhury EK, et al. (July 2003). “Dietary mushroom (Pleurotus ostreatus) ameliorates atherogenic lipid in hypercholesterolaemic rats”. Clin Exp Pharmacol Physiol. 30(7): 470–5. doi:10.1046/j.1440-1681.2003.03857.x.PMID 12823261.
- Jump up^ Bobek P, Galbavý S (October 1999). “Hypocholesterolemic and antiatherogenic effect of oyster mushroom (Pleurotus ostreatus) in rabbits”. Nahrung. 43 (5): 339–42. doi:10.1002/(SICI)1521-3803(19991001)43:5<339::AID-FOOD339>3.0.CO;2-5.PMID 10555301.
- Jump up^ Opletal L, Jahodár L, Chobot V, et al. (December 1997). “Evidence for the anti-hyperlipidaemic activity of the edible fungus Pleurotus ostreatus”. Br. J. Biomed. Sci. 54 (4): 240–3. PMID 9624732.
- Jump up^ Bajaj M, Vadhera S, Brar AP, Soni GL (October 1997). “Role of oyster mushroom (Pleurotus florida) as hypocholesterolemic/antiatherogenic agent”. Indian J. Exp. Biol. 35(10): 1070–5. PMID 9475042.
- Jump up^ Bobek P, Ozdín L, Kuniak L, Hromadová M (March 1997). “[Regulation of cholesterol metabolism with dietary addition of oyster mushrooms (Pleurotus ostreatus) in rats with hypercholesterolemia]”.Cas. Lek. Cesk. (in Slovak). 136 (6): 186–90. PMID 9221192.
- Jump up^ Bobek P, Ozdín L, Kuniak L (August 1996). “Effect of oyster mushroom (Pleurotus Ostreatus) and its ethanolic extract in diet on absorption and turnover of cholesterol in hypercholesterolemic rat”.Nahrung. 40 (4): 222–4. doi:10.1002/food.19960400413.PMID 8810086.
- Jump up^ Bobek P, Ozdín O, Mikus M (1995). “Dietary oyster mushroom (Pleurotus ostreatus) accelerates plasma cholesterol turnover in hypercholesterolaemic rat”. Physiol Res. 44 (5): 287–91.PMID 8869262.
- Jump up^ Bobek P, Ozdin L, Kuniak L (1995). “The effect of oyster mushroom (Pleurotus ostreatus), its ethanolic extract and extraction residues on cholesterol levels in serum, lipoproteins and liver of rat”. Nahrung. 39(1): 98–9. doi:10.1002/food.19950390113. PMID 7898579.
- Jump up^ Bobek P, Ozdin L, Kuniak L (March 1994). “Mechanism of hypocholesterolemic effect of oyster mushroom (Pleurotus ostreatus) in rats: reduction of cholesterol absorption and increase of plasma cholesterol removal”. Z Ernahrungswiss. 33 (1): 44–50.doi:10.1007/BF01610577. PMID 8197787.
- Jump up^ Chorváthová V, Bobek P, Ginter E, Klvanová J (1993). “Effect of the oyster fungus on glycaemia and cholesterolaemia in rats with insulin-dependent diabetes”. Physiol Res. 42 (3): 175–9.PMID 8218150.
- Jump up^ Bobek P, Ginter E, Jurcovicová M, Kuniak L (1991). “Cholesterol-lowering effect of the mushroom Pleurotus ostreatus in hereditary hypercholesterolemic rats”. Ann. Nutr. Metab. 35 (4): 191–5.doi:10.1159/000177644. PMID 1897899.
- Jump up^ Khatun K, Mahtab H, Khanam PA, Sayeed MA, Khan KA (January 2007). “Oyster mushroom reduced blood glucose and cholesterol in diabetic subjects”. Mymensingh Med J. 16 (1): 94–9.doi:10.3329/mmj.v16i1.261. PMID 17344789.
- Jump up^ “FDA bans red yeast rice product” by Michael McCarthy, The Lancet, Volume 351, Issue 9116, Page 1637, 30 May 1998
- Jump up^ Cholesterol Treatment Upheld, The New York Times, February 18, 1999
- Jump up^ Coronary heart disease: MedLine Plus Medical Encyclopedia
- Jump up^ Endo, Akira; Kuroda M.; Tsujita Y. (December 1976). “ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium”. Journal of Antibiotics (Tokyo). 29(12): 1346–8. doi:10.7164/antibiotics.29.1346. PMID 1010803.
- Jump up^ Witter, DJ; Vederas, JC (1996). “Putative Diels-Alder catalyzed cyclization during the biosynthesis of lovastatin”. J Org Chem. 61 (8): 2613–23. doi:10.1021/jo952117p. PMID 11667090.
- Jump up^ Hirama M, Vet M (1982). “A chiral total synthesis of compactin”. J. Am. Chem. Soc. 104 (15): 4251. doi:10.1021/ja00379a037.
- Jump up^ Hirama M, Iwashita; Iwashita, Mitsuko (1983). “Synthesis of (+)-Mevinolin starting from Naturally occurring building blocks and using an asymmetry inducing reaction”. Tetrahedron Lett. 24 (17): 1811–1812. doi:10.1016/S0040-4039(00)81777-3.
- Jump up^ Javernik S, Kreft S, Strukelj B, Vrecer F (2001). “Oxidation of lovastatin in the solid state and its stabilization with natural antioxidants”. Die Pharmazie. 56 (9): 738–40. PMID 11593996.
- Jump up^ Hartig K, Beck E (2005). “Assessment of lovastatin application as tool in probing cytokinin-mediated cell cycle regulation”. Physiologia Plantarum. 125 (2): 260–267. doi:10.1111/j.1399-3054.2005.00556.x.
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
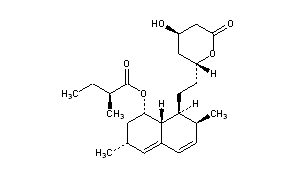
(1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-Hydroxy-6-oxooxan-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate
|
|
| Clinical data | |
| Trade names | Mevacor |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a688006 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | <5%[1] |
| Protein binding | >98%[1] |
| Metabolism | Hepatic (CYP3A andCYP2C8 substrate)[1] |
| Biological half-life | 2–5 hours[1] |
| Excretion | Faeces (83%), urine (10%)[1] |
| Identifiers | |
| CAS Number | 75330-75-5 |
| ATC code | C10AA02 (WHO) |
| PubChem | CID 53232 |
| IUPHAR/BPS | 2739 |
| DrugBank | DB00227 |
| ChemSpider | 48085 |
| UNII | 9LHU78OQFD |
| KEGG | D00359 |
| ChEBI | CHEBI:40303 |
| ChEMBL | CHEMBL503 |
| Synonyms | Monacolin K, Mevinolin |
| Chemical data | |
| Formula | C24H36O5 |
| Molar mass | 404.54 g/mol |
////////////////

















Sorry, the comment form is closed at this time.