|
Title: Acetazolamide
CAS Registry Number: 59-66-5
CAS Name: N-[5-(Aminosulfonyl)-1,3,4-thiadiazol-2-yl]acetamide
Additional Names: 5-acetamido-1,3,4-thiadiazole-2-sulfonamide; 2-acetylamino-1,3,4-thiadiazole-5-sulfonamide
Manufacturers’ Codes: 6063
Trademarks: Acetamox (Tobishi-Santen); Atenezol (Tsuruhara); Défiltran (Gallier); Diamox (Barr); Didoc (Sawai); Diuriwas (IFI); Donmox (Horita); Edemox (Wassermann); Fonurit (Chinoin); Glaupax (Erco)
Molecular Formula: C4H6N4O3S2
Molecular Weight: 222.25
Percent Composition: C 21.62%, H 2.72%, N 25.21%, O 21.60%, S 28.85%
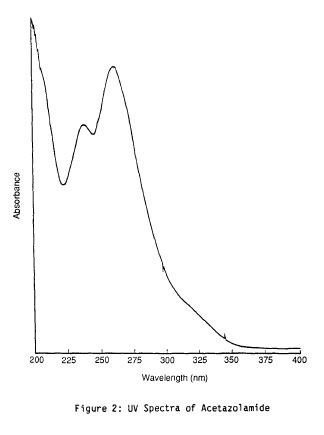
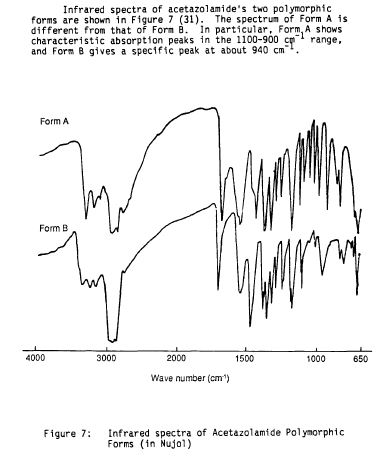
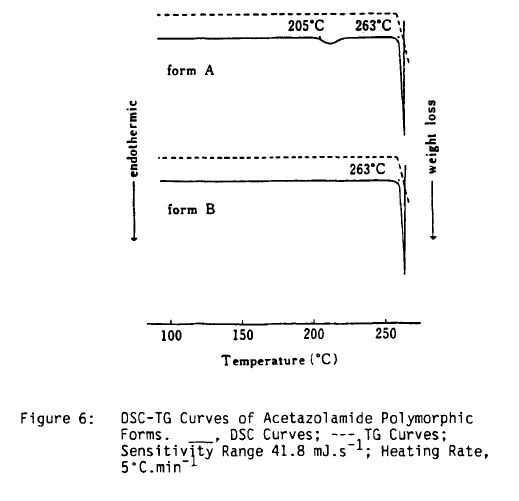
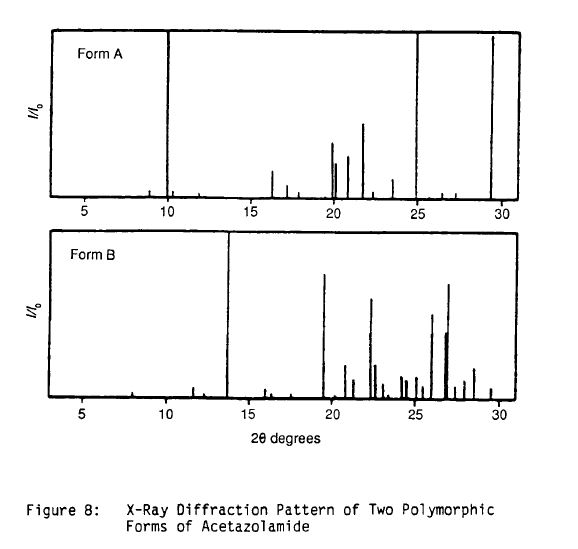
Literature References: Carbonic anhydrase inhibitor. Prepn: R. O. Roblin, J. W. Clapp, J. Am. Chem. Soc. 72, 4890 (1950); J. W. Clapp, R. O. Roblin, US 2554816 (1951 to Am. Cyanamid). HPLC determn in pharmaceuticals: Z. S. Gomaa, Biomed. Chromatogr. 7, 134 (1993). Effect on retinal circulation: S. M. B. Rassam et al., Eye 7, 697 (1993). Clinical trial in postoperative elevation of intraocular pressure: I. D. Ladas et al., Br. J. Ophthalmol. 77, 136 (1993). Comprehensive description: J. Parasrampuria, Anal. Profiles Drug Subs. Excip. 22, 1-32 (1993). Review of efficacy in acute mountain sickness: L. D. Ried et al.,J. Wilderness Med. 5, 34-48 (1994).
Properties: Crystals from water, mp 258-259° (effervescence). Weak acid. pKa 7.2. Sparingly sol in cold water. Slightly sol in alcohol, acetone. Practically insol in carbon tetrachloride, chloroform, ether. Soly (mg/ml): polyethylene glycol-400 87.81; propylene glycol 7.44; ethanol 3.93; glycerin 3.65; water 0.72.
Melting point: mp 258-259° (effervescence)
pKa: pKa 7.2
Derivative Type: Sodium salt
CAS Registry Number: 1424-27-7
Trademarks: Vetamox (Am. Cyanamid)
Therap-Cat: Antiglaucoma; diuretic; in treatment of acute mountain sickness.
Therap-Cat-Vet: Diuretic.
Keywords: Antiglaucoma; Carbonic Anhydrase Inhibitor; Diuretic; Sulfonamide Derivatives.
|



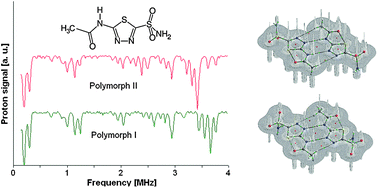
1H NMR
14N NQR, 1H NMR and DFT/QTAIM study of hydrogen bonding and polymorphism in selected solid 1,3,4-thiadiazole derivatives
E-mail: janez.seliger@fmf.uni-lj.si
Fax: +386 1 2517281
Tel: +386 1 4766576
DOI: 10.1039/C0CP00195C, http://pubs.rsc.org/en/content/articlelanding/2010/cp/c0cp00195c#!divAbstract
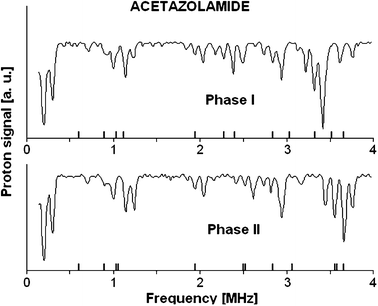
The 1,3,4-thiadiazole derivatives (2-amino-1,3,4-thiadiazole, acetazolamide, sulfamethizole) have been studied experimentally in the solid state by 1H–14N NQDR spectroscopy and theoretically by Density Functional Theory (DFT). The specific pattern of the intra and intermolecular interactions in 1,3,4-thiadiazole derivatives is described within the QTAIM (Quantum Theory of Atoms in Molecules)/DFT formalism. The results obtained in this work suggest that considerable differences in the NQR parameters permit differentiation even between specific pure association polymorphic forms and indicate that the stronger hydrogen bonds are accompanied by the larger η and smaller ν− and e2Qq/h values. The degree of π-electron delocalization within the 1,3,4-thiadiazole ring and hydrogen bonds is a result of the interplay between the substituents and can be easily observed as a change in NQR parameters at N atoms. In the absence of X-ray data NQR parameters can clarify the details of crystallographic structure revealing information on intermolecular interactions.
////////////ацетазоламид , أسيتازولاميد [, 乙酰唑胺 , ACETAZOLAMIDE
CC(=O)NC1=NN=C(S1)S(N)(=O)=O














Sorry, the comment form is closed at this time.