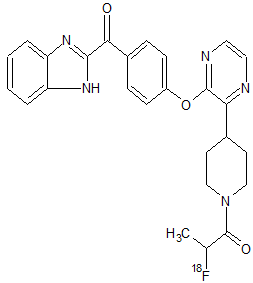
[18F]AMG 580
NOTE………CAS OF AMG 580 IS 1227067-71-1, WITHOUT 18F
AMG 580 [1-(4-(3-(4-(1H-benzo[d]imidazole-2-carbonyl)phenoxy)pyrazin-2-yl)piperidin-1-yl)-2-fluoropropan-1-one],
Phosphodiesterase 10A (PDE10A) inhibitors have therapeutic potential for the treatment of psychiatric and neurologic disorders, such as schizophrenia and Huntington’s disease. One of the key requirements for successful central nervous system drug development is to demonstrate target coverage of therapeutic candidates in brain for lead optimization in the drug discovery phase and for assisting dose selection in clinical development. Therefore, we identified AMG 580 [1-(4-(3-(4-(1H-benzo[d]imidazole-2-carbonyl)phenoxy)pyrazin-2-yl)piperidin-1-yl)-2-fluoropropan-1-one], a novel, selective small-molecule antagonist with subnanomolar affinity for rat, primate, and human PDE10A. We showed that AMG 580 is suitable as a tracer for lead optimization to determine target coverage by novel PDE10A inhibitors using triple-stage quadrupole liquid chromatography–tandem mass spectrometry technology. [3H]AMG 580 bound with high affinity in a specific and saturable manner to both striatal homogenates and brain slices from rats, baboons, and human in vitro. Moreover, [18F]AMG 580 demonstrated prominent uptake by positron emission tomography in rats, suggesting that radiolabeled AMG 580 may be suitable for further development as a noninvasive radiotracer for target coverage measurements in clinical studies. These results indicate that AMG 580 is a potential imaging biomarker for mapping PDE10A distribution and ensuring target coverage by therapeutic PDE10A inhibitors in clinical studies.
PAPER

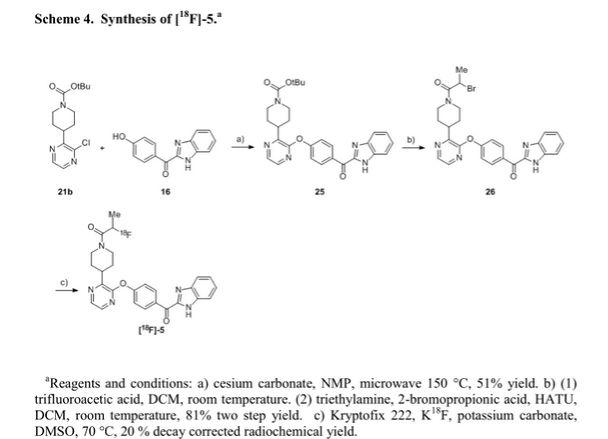
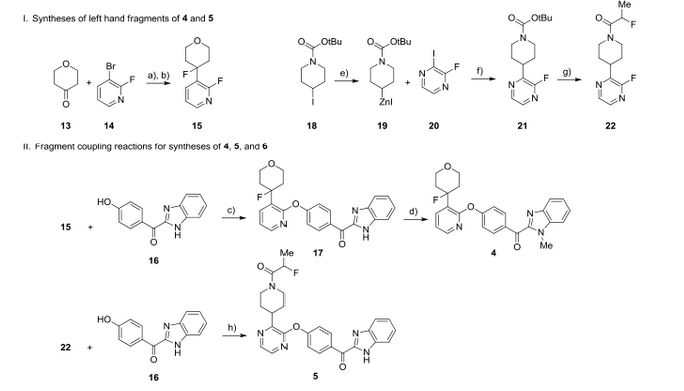
We report the discovery of PDE10A PET tracer AMG 580 developed to support proof of concept studies with PDE10A inhibitors in the clinic. To find a tracer with higher binding potential (BPND) in NHP than our previously reported tracer 1, we implemented a surface plasmon resonance assay to measure the binding off-rate to identify candidates with slower washout rate in vivo. Five candidates (2–6) from two structurally distinct scaffolds were identified that possessed both the in vitro characteristics that would favor central penetration and the structural features necessary for PET isotope radiolabeling. Two cinnolines (2, 3) and one keto-benzimidazole (5) exhibited PDE10A target specificity and brain uptake comparable to or better than 1 in the in vivo LC–MS/MS kinetics distribution study in SD rats. In NHP PET imaging study, [18F]-5 produced a significantly improved BPND of 3.1 and was nominated as PDE10A PET tracer clinical candidate for further studies.
Discovery of Phosphodiesterase 10A (PDE10A) PET Tracer AMG 580 to Support Clinical Studies
PATENT FOR AMG 580
WO 2010057121
https://www.google.com/patents/WO2010057121A1?cl=en
PAPER
Nuclear Medicine and Biology (2015), 42(8), 654-663.
http://www.sciencedirect.com/science/article/pii/S0969805115000724
Phosphodiesterase 10A (PDE10A) is an intracellular enzyme responsible for the breakdown of cyclic nucleotides which are important second messengers for neurotransmission. Inhibition of PDE10A has been identified as a potential target for treatment of various neuropsychiatric disorders. To assist drug development, we have identified a selective PDE10A positron emission tomography (PET) tracer, AMG 580. We describe here the radiosynthesis of [18 F]AMG 580 and in vitro and in vivo characterization results.
AMG 580 has an in vitro KD of 71.9 pM. Autoradiography showed specific uptake in striatum. Mean activity of 121 ± 18 MBq was used in PET studies. In Rhesus, the baseline BPND for putamen and caudate was 3.38 and 2.34, respectively, via 2TC, and 3.16, 2.34 via Logan, and 2.92, and 2.01 via SRTM. A dose dependent decrease of BPNDwas observed by the pre-treatment with a PDE10A inhibitor. In baboons, 0.24 mg/kg dose of AMG 580 resulted in about 70% decrease of BPND. The in vivo KD of [18 F]AMG 580 was estimated to be around 0.44 nM in baboons.
Conclusion
[18 F]AMG 580 is a selective and potent PDE10A PET tracer with excellent specific striatal binding in non-human primates. It warrants further evaluation in humans.
REFERNCES
http://jpet.aspetjournals.org/content/352/2/327.full
///Phosphodiesterase, tracer, receptor occupancy, positron emission tomography, radiotracer, brain penetration, AMG 580, Phosphodiesterase 10A, PDE10A, PET Tracer, [18F]AMG 580













Sorry, the comment form is closed at this time.