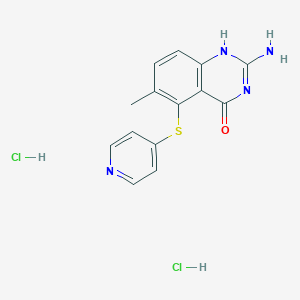
Nolatrexed
NDA Filed in china
A thymidylate synthase inhibitor potentially for the treatment of hepatocellular carcinoma and nasopharyngeal cancer.
![]()
AG-337
CAS No. 147149-76-6 (free)
free form data
(eluents: CH3CN−H2O = 10−90, pH 4.94; Rt = 11.8 min); Rf = 0.31 [ethyl acetate/(0.63 M NH3 in ethanol) = 6/4]; Mp 300−302 °C (lit.:(J. Med. Chem. 1993, 36, 733– 746) a tan solid; Mp 301−302 °C); MS (ESI+) m/z: 285.1 [M + 1]+; the major impurity: 3.0% (Rt = 13.0 min); Mp 73−77 °C; 1H NMR (DMSO-d6): δ 7.95 (d, J = 6.4 Hz, 4 H), 8.81 (d, J = 6.4 Hz, 4 H);
MS (ESI+) m/z: 219.2 [M − 1]+;
152946-68-4(Nolatrexed Dihydrochloride)
2-amino-6-methyl-5-pyridin-4-ylsulfanyl-1H-quinazolin-4-one;dihydrochloride
| Molecular Formula: | C14H14Cl2N4OS |
|---|---|
| Molecular Weight: | 357.25816 g/mol |
diHCl data
IR (KBr cm−1):ṽ 3401, 3058, 2929, 1701, 1621, 1471, 799;
1H NMR (DMSO-d6): δ 2.43 (s, 3H, −CH3), 7.53 (d,J = 6.9 Hz, 2H, Pyr-H), 7.67 (d, J = 8.5 Hz, 1H, Ar−H), 7.92 (d, J = 8.5 Hz, 1 Hz, Ar−H), 8.30 (br s, 3H, NH3), 8.52 (d, J = 6.9 Hz, 2H, Pyr-H); MS (ESI+) m/z: 285 [M − 1−2Cl]+; (ESI+) m/z: 283 [M − 1− 2HCl]+.
Pfizer (Originator) , Gilead,LG Life Sciences,北京康辰药业
Nolatrexed is a thymidylate synthase inhibitor.[1][2]
Phase I studies of p.o. administered nolatrexed dihydrochloride (AG337, THYMITAQ), a nonclassical thymidylate synthase inhibitor, were performed to establish the maximum tolerated dose and a recommended dose for Phase II studies. The bioavailability and pharmacokinetic and pharmacodynamic properties of oral nolatrexed were also studied. Forty-five patients were treated with oral nolatrexed every 6 h for 5 days at doses of 288-1000 mg/m2/day. The bioavailability of the oral preparation was determined, and the effect of a standard meal on nolatrexed absorption was investigated at a dose of 800 mg/m2/day. Nolatrexed plasma concentrations were analyzed by high-performance liquid chromatography. Nolatrexed was rapidly absorbed with a median bioavailability of 89% (range 33-116%), with 88% of patients above 70%. The dose-limiting toxicities were gastrointestinal, and the recommended Phase II oral dose was 800 mg/m2/day. After a standard meal, the peak plasma nolatrexed concentration achieved was lower (median, 8.3 microg/ml versus 15.0 microg/ml; P = 0.001), and the time taken to reach the peak was longer (median, 180 min versus 45 min; P = 0.00003), but the trough concentration was higher (median, 3.6 microg/ml versus 2.1 microg/ml; P = 0.004) when compared with the fasted state. The area under the nolatrexed plasma concentration versus time curve was not affected by food. Average trough nolatrexed concentration, but not dose, was significantly related to the % decrease in both thrombocytes (r2 = 0.58; C50 = 6.0 microg/ml, where C50 is the plasma concentration associated with a 50% decrease in thrombocytes) and neutrophils (r2 = 0.63; C50 = 0.6 microg/ml). Nolatrexed can be safely administered as an oral preparation at a dose of 800 mg/m2/day for 5 days. Bioavailability was close to 100% and, because inhibition of thymidylate synthase by nolatrexed is rapidly reversible, the slower absorption after a standard meal may result in a shorter duration of noninhibitory concentrations between doses.

Catalytic hydrogenation of 2-bromo-4 -nitrotoluene (I) over Raney-Ni provided aniline (II). Reaction of (II) with chloral hydrate and hydroxylamine gave rise to the isonitrosoacetanilide (III), which was subsequently cyclized to the isatin (IV) by heating in concentrated H2SO4. Oxidative cleavage of isatin (IV) produced the anthranilic acid (V). This was converted to the benzoxazinone (VI) upon refluxing with acetic anhydride. Ring opening of benzoxazinone (VI) with MeOH, followed by acidic hydrolysis of the acetamide function, yielded the anthranilate ester (VII). The quinazoline derivative (VIII) was then obtained by treatment of anthranilate (VII) with chloroformamidine hydrochloride in refluxing diglyme. Finally, displacement of the bromide group of (VIII) with the sodium thiolate of 4-mercaptopyridine (IX) under Ullmann conditions afforded the title pyridyl sulfide.
| Dissertation title | [BT] A New Method for Synthesis of Nolatrexed Dihydrochloride | ||
| Hangul title | Nolatrexed dihydrochloride Synthesis Process Development | ||
| Author | Xueqing Zhao, Fei Li, Weiping Zhuang, Xiaowen Xue, Yuanyang Lian, Jianhui Fan and Dongsheng Fang | ||
| Japjimyeong | ORG PROCESS RES DEV | Issue year | 2010 |
| Gwonho details | 14 (2) | The surface | 346-350 |
| ABSTRACT |
.bmp) A new synthetic method for nolatrexed dihydrochloride (thymitaq) has been developed. The synthesis was accomplished in three steps featuring the direct conversion of the starting 4-bromo-5-methylisatin into the methyl anthranilate by potassium peroxydisulfate / sodium methoxide. In the final Ullmann reaction potassium carbonate was employed in place of sodium hydride, and the amount of copper catalysts was significantly reduced. Moreover, sodium sulfide solution was utilized to efficiently remove copper under approximately neutral conditions instead of hydrogen sulfide / methanol under strongly acidic conditions. By means of these modifications, nolatrexed dihydrochloride was ensured to be prepared in good yield and high purity.
|
||
| Contents |
Nolatrexed dihydrochloride (2-Amino-6-methyl-5-(4-pyridylthio) -3 H-quinazolin-4-one dihydrochloride, thymitag, 1) is the HCC cancer therapeutic agent to the TS (thymidylate synthase) folate binding site on the TS inhibitor as DNA replication inhibition, DNA damage, S-phase cell cycle arrest, and caspase-dependent apoptosis induction and clinical 2 on theresults look HCC patients, the survival benefit of showing the current phase III study is in progress in it. under scheme 1 is conducted in a number of synthesis team Nolatrexedillustrates the development process Scheme 1. Synthetic routes A-F from 4-bromo-5-methylisatin (2) to nolatrexed dihydrochloride (1)
.bmp) The scheme 1 When the complex first synthesis process but is A : 2 – 3 – 4 – 5 – 7 · HCl – 1 or in part, 6 pass through a B step ( 2 – 3 – 6 – 5 ) to obtain the desired compound with, but However, these processes are of the desired product quality control had a disadvantage unfulfilled this . after C, D, E process was developed during the E step is a step wherein compound 8 from the first to the one-pot is the most superior process consists in the process also drug of the compound for use as a quality control has difficulty in . more recentlyWennerberg is a new process F compounds were reported for 3 compound directly from the 7fully in the process I scored quality control could be the place . in the process, each reactionstep partially changed by the use of a reagent zoom impurity to minimize the formation of .However, this process also work-up, and purification there have difficulties to process the authors reported a new efficient way .
Scheme 2. Synthetic route G from 4-bromo-5-methylisatin (2) to nolatrexed dihydrochloride (1)
.gif) Scheme 2 The process reported to also have specifically not a new process only takes the best features from several processes previously reported , significant differences that the author is proud director teen two direct compound from 5 will get the , also reported in other processes already advanced mercaptopyridine introducing Ullmann reaction in the processimpurity , to reduce the formation of NaH , instead of K2CO3 were used the copper catalyst in order to minimize the amount of copper scavenge used to H2S instead of Na2S was used . the compound obtained in the process 1 of the purity is 96.6% and 3% with impurities of the 4,4′-dithiodipyridine this was confirmed copper impurity is 20 ppm was below . last Nolatrexed dihydrochloride in the process to obtain a 99.7% purity I scored the desired product , 0.3% ofunidentified impurity, and 10 ppm less than copper because it contains should think very advanced process compared to the previous number of ways . Fortunately Ullmann key contained in the reaction impurity in 4,4′-dithiodipyridine was automatically removed from the crystallization process of the last reaction.
Korea Research Institute of Chemical Technology provides incurable disease treatment and research center, Dr. jaedu |
||
| View original | http://pubs.acs.org/doi/full/10.1021/op9002517 | ||
References
- Hughes AN, Rafi I, Griffin MJ, et al. (January 1999). “Phase I studies with the nonclassical antifolate nolatrexed dihydrochloride (AG337, THYMITAQ) administered orally for 5 days”. Clin. Cancer Res. 5 (1): 111–8. PMID 9918208.
- “Nolatrexed”. PubChem.gov. Pub Chem. Retrieved 12 August 2014.
 |
|
| Names | |
|---|---|
| IUPAC name
2-Amino-6-methyl-5-(4-pyridylthio)-1H-quinazolin-4-one
|
|
| Identifiers | |
| 147149-76-6 |
|
| ChemSpider | 97268 |
| Jmol 3D model | Interactive image |
| PubChem | 108189 |
| UNII | K75ZUN743Q |
| Properties | |
| C14H12N4OS | |
| Molar mass | 284.34 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|