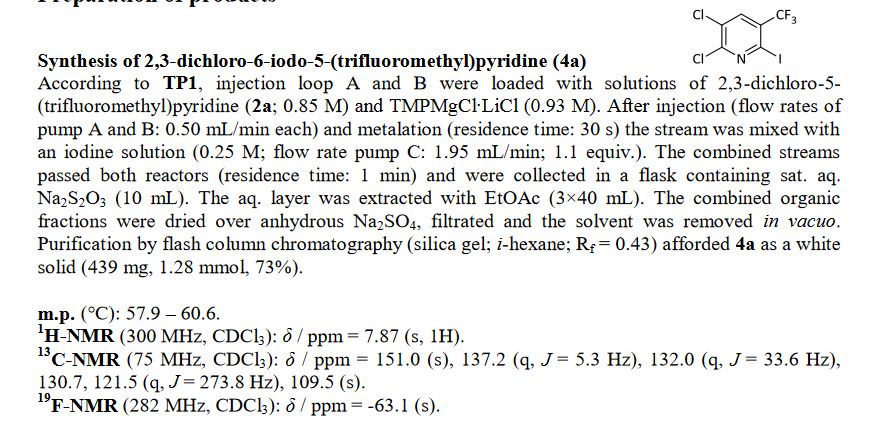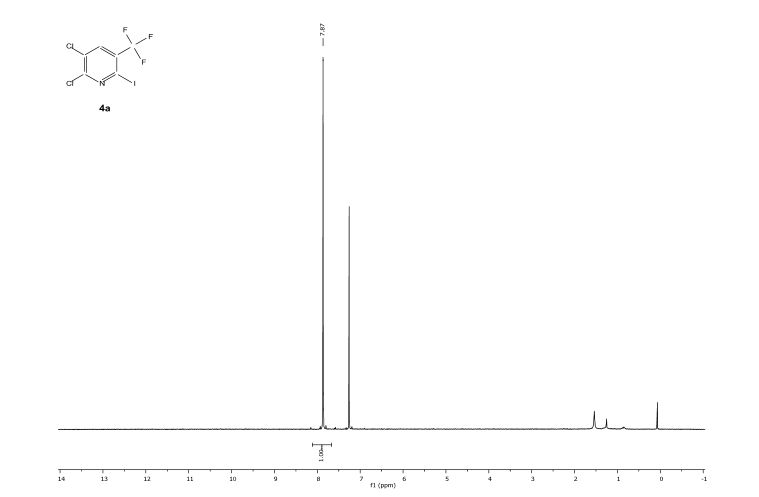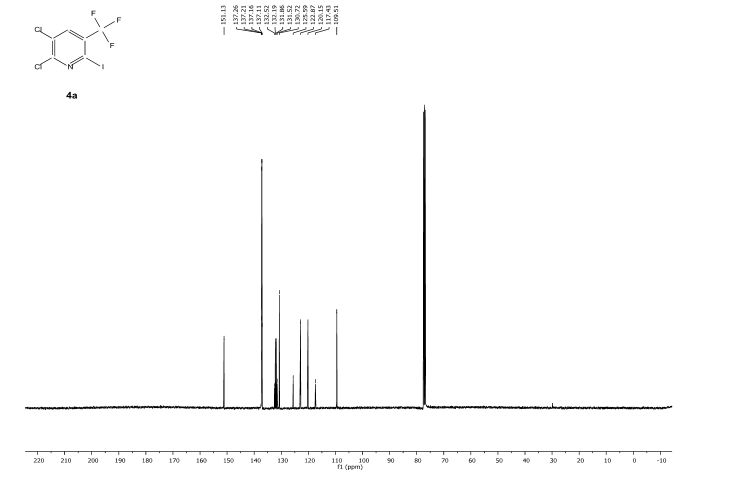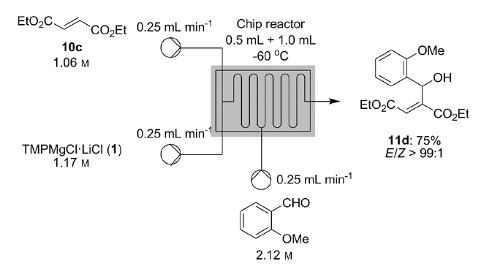
Knochel’s group in Munich have recently disclosed how a variety of functionalised heterocycles and sensitive acrylates can be rapidly magnesiated and subsequently quenched with an electrophile under continuous flow-through;conditions using a Uniqsis static mixer/reactor chip.
A key advantage is that, in contrast to typical batch procedures, these reactions required non-cryogenic conditions (typically 25C); moreover the procedure could be quickly scaled to 45 mmol without modification of the reaction conditions.
Metalations under flow-through conditions permited magnesiations that did not afford the desired product under batch conditions and acylates could be magnesiated and quenched to afford products with high stereoselectivities without concomitant polymerisation.
Continuous Flow Magnesiation of Functionalized Heterocycles and Acrylates with TMPMgCl⋅LiCl†
A flow procedure for the metalation of functionalized heterocycles (pyridines, pyrimidines, thiophenes, and thiazoles) and various acrylates using the strong, non-nucleophilic base TMPMgCl⋅LiCl is reported. The flow conditions allow the magnesiations to be performed under more convenient conditions than the comparable batch reactions, which often require cryogenic temperatures and long reaction times. Moreover, the flow reactions are directly scalable without further optimization. Metalation under flow conditions also allows magnesiations that did not produce the desired products under batch conditions, such as the magnesiation of sensitive acrylic derivatives. The magnesiated species are subsequently quenched with various electrophiles, thereby introducing a broad range of functionalities.
see
http://onlinelibrary.wiley.com/doi/10.1002/anie.201404221/full
/////Continuous Flow Magnesiation, Functionalized Heterocycles, Acrylates , TMPMgCl⋅LiCl