Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis have shown venlafaxine, alongside
mirtazapine,
escitalopram and
sertraline were significantly more efficacious.
[8] Remission rates (defined as a HAM-D score of 7 or less) were 58% for venlafaxine plus mirtazapine.
[9]The rate of life-threatening or lethal outcomes for suicidal overdoses of venlafaxine is lower than for the
TCAs,
MAOIs and
bupropionand comparable to several of the
SSRIs.
[10] It is metabolised in the body into another antidepressant drug called
desvenlafaxine (
O-desmethylvenlafaxine) which is also sold as an antidepressant, under the brand name Pristiq.
[11]Both venlafaxine and
paroxetine have been linked to the most severe discontinuation symptomes.
In 2007, venlafaxine was the sixth most commonly prescribed antidepressant on the U.S. retail market, with 17.2 million prescriptions.
[12]
Chemistry
The
chemical structure of venlafaxine is designated (R/S)-1-[2-(dimethylamino)-1-(4 methoxyphenyl)ethyl] cyclohexanol hydrochloride or (±)-1-[a [a- (dimethylamino)methyl] p-methoxybenzyl] cyclohexanol hydrochloride, and it has the
empirical formula of C
17H
27NO
2. It is a white to off-white crystalline solid. Venlafaxine is structurally and pharmacologically related to the atypical opioid
analgesictramadol, and more distantly to the newly released opioid
tapentadol, but not to any of the conventional antidepressant drugs, including
tricyclic antidepressants, SSRIs, MAOIs, or
RIMAs.
[66]

Derivative Type: Hydrochloride
CAS : 99300-78-4
Manufacturers’ Codes: Wy-45030
Trademarks: Effexor (Wyeth)
Molecular Formula: C17H27NO2.HCl
Molecular Weight: 313.86
Percent Composition: C 65.06%, H 8.99%, N 4.46%, O 10.20%, Cl 11.30%
Properties: White to off-white crystalline solid from methanol/ethyl acetate, mp 215-217°. Soly (mg/ml): 572 water. Partition coefficient (octanol/water): 0.43.
Melting point: mp 215-217°
Log P: Partition coefficient (octanol/water): 0.43
Derivative Type: (+)-Form
Properties: Crystals from ethyl acetate, mp 102-104°. [a]D25 +27.6° (c = 1.07 in 95% ethanol).
Melting point: mp 102-104°
Optical Rotation: [a]D25 +27.6° (c = 1.07 in 95% ethanol)
Derivative Type: (+)-Form hydrochloride
Manufacturers’ Codes: Wy-45655
Properties: Crystals from methanol/ether, mp 240-240.5°. [a]D25 -4.7° (c = 0.945 in ethanol).
Melting point: mp 240-240.5°
Optical Rotation: [a]D25 -4.7° (c = 0.945 in ethanol)
Derivative Type: (-)-Form
Properties: Crystals from ethyl acetate, mp 102-104°. [a]D25 -27.1° (c = 1.04 in 95% ethanol).
Melting point: mp 102-104°
Optical Rotation: [a]D25 -27.1° (c = 1.04 in 95% ethanol)
Derivative Type: (-)-Form hydrochloride
Manufacturers’ Codes: Wy-45651
Properties: Crystals from methanol/ether, mp 240-240.5°. [a]D25 +4.6° (c = 1.0 in ethanol).
Melting point: mp 240-240.5°
Optical Rotation: [a]D25 +4.6° (c = 1.0 in ethanol)
Therap-Cat: Antidepressant.
Keywords: Antidepressant; Serotonin Noradrenaline Reuptake Inhibitor (SNRI).
1H NMR
HSQC























1H NMR PREDICT OF HCL

13C NMR PREDICT OF HCL

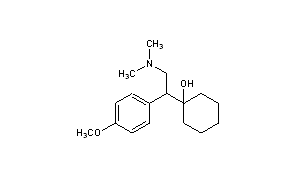
BASE


Literature References:
Serotonin noradrenaline reuptake inhibitor (SNRI). Prepn: G. E. M. Husbands et al., EP 112669; US4535186 (1984, 1985 both to Am. Home Prods.);
and resolution of isomers: J. P. Yardley et al., J. Med. Chem. 33, 2899 (1990). Receptor binding studies: E. A. Muth et al., Biochem. Pharmacol. 35, 4493 (1986).
HPLC determn in biological fluids: D. R. Hickset al., Ther. Drug Monit. 16, 100 (1994).
Clinical pharmacokinetics: K. J. Klamerus et al., J. Clin. Pharmacol. 32, 716 (1992).
Clinical trial in major depression: E. Schweizer et al., J. Clin. Psychopharmacol. 11, 233 (1991).
Review of pharmacology and clinical efficacy in depression: S. A. Montgomery, J. Clin. Psychiatry 54, 119-126 (1993).
Clinical trial in generalized anxiety disorder: A. J. Gelenberg et al., J. Am. Med. Assoc. 283, 3082 (2000).
External links[Drug information
Diagnostic tools
Patient experiences
P.S.
: The views expressed are my personal and in no-way suggest the views
of the professional body or the company that I represent.
P.S.
: The views expressed are my personal and in no-way suggest the views
of the professional body or the company that I represent.
P.S.
: The views expressed are my personal and in no-way suggest the views
of the professional body or the company that I represent.


COCK WILL TEACH YOU NMR
 COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR

Join me on twitter

 amcrasto@gmail.com
amcrasto@gmail.com

 2014, Vol. 35
2014, Vol. 35  Issue (12): 2584-2592 DOI: 10.7503/cjcu20140333
Issue (12): 2584-2592 DOI: 10.7503/cjcu20140333














 SEE
SEE











 COCK WILL TEACH YOU NMR
COCK WILL TEACH YOU NMR COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR