Takasago has been devoted to producing l-menthol since 1954, and our long history of manufacturing this important aroma chemical is reviewed here. The current asymmetric catalytic process had its 30th anniversary in 2013. Our l-menthol process is considered carbon-neutral, and, therefore, ‘green’ and sustainable. It uses renewable myrcene obtained from gum rosin as a starting material. In addition, the Rh-BINAP (=2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) catalytic system is highly efficient. This pathway not only leads l-menthol, but a variety of 100% biobased aroma chemical products as well. By measuring the 14C levels in a material, one can determine the percentage of carbon that is biobased. This biobased assay, described as the ratio plant-derived C/fossil-derived C, can clarify how renewable a product really is. This will be highlighted for several of Takasago’s key aroma chemicals.
A Green and Sustainable Approach: Celebrating the 30th Anniversary of the Asymmetric l-Menthol Process
Article first published online: 18 NOV 2014
DOI: 10.1002/cbdv.201400063
Issue
1612-1880/asset/cover.gif?v=1&s=cc68b12e733d21d6415eeec10551f83cb03996eb)
Chemistry & Biodiversity
Volume 11, Issue 11, pages 1688–1699, November 2014
http://onlinelibrary.wiley.com/doi/10.1002/cbdv.201400063/abstract
Production
As with many widely used natural products, the demand for menthol greatly exceeds the supply from natural sources. In the case of menthol it is also interesting to note that comparative analysis of the total life-cycle costs from a sustainability perspective, has shown that production from natural sources actually results in consumption of more fossil fuel, produces more carbon dioxide effluent and has more environmental impact than either of the main synthetic production routes.[7]
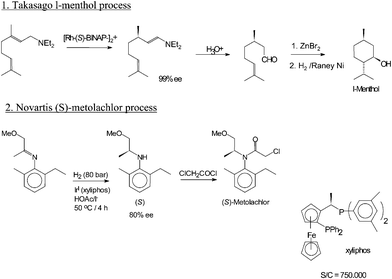
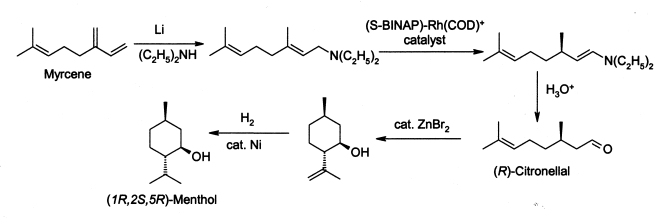
Menthol is manufactured as a single enantiomer (94% ee) on the scale of 3,000 tons per year by Takasago International Corporation.[8] The process involves an asymmetric synthesis developed by a team led by Ryōji Noyori, who won the 2001 Nobel Prize for Chemistry in recognition of his work on this process:
The process begins by forming an allylic amine from myrcene, which undergoes asymmetric isomerisation in the presence of a BINAP rhodium complex to give (after hydrolysis) enantiomerically pure R–citronellal. This is cyclised by a carbonyl-ene-reaction initiated by zinc bromide to isopulegol, which is then hydrogenated to give pure (1R,2S,5R)-menthol.
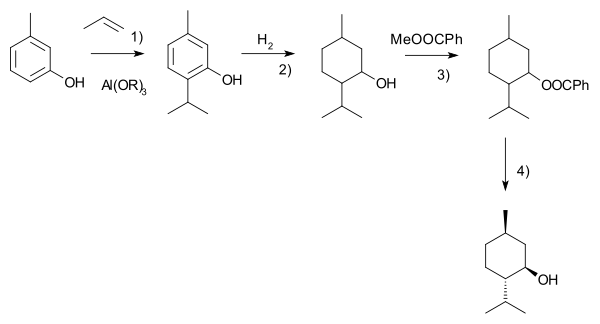
Another commercial process is the Haarmann-Reimer process. [9][10] This process starts from m-cresol which is alkylated with propene to thymol. This compound is hydrogenatedin the next step. Racemic menthol is isolated by fractional distillation. The enantiomers are separated by chiral resolution in reaction with methyl benzoate, selective crystallisation followed by hydrolysis.
Racemic menthol can also be formed by hydrogenation of pulegone. In both cases with further processing (crystallizative entrainment resolution of the menthyl benzoate conglomerate) it is possible to concentrate the L enantiomer, however this tends to be less efficient, although the higher processing costs may be offset by lower raw material costs. A further advantage of this process is that d-menthol becomes inexpensively available for use as a chiral auxiliary, along with the more usual l-antipode.[7]
References
- R. Eccles (1994). “Menthol and Related Cooling Compounds”. J. Pharm. Pharmacol. 46 (8): 618–630. PMID 7529306.
- Galeottia, N., Mannellia, L. D. C., Mazzantib, G., Bartolinia, A., Ghelardini, C.; Di Cesare Mannelli; Mazzanti; Bartolini; Ghelardini (2002). “Menthol: a natural analgesic compound”.Neuroscience Letters 322 (3): 145–148. doi:10.1016/S0304-3940(01)02527-7. PMID 11897159.
- G. Haeseler, D. Maue, J. Grosskreutz, J. Bufler, B. Nentwig, S. Piepenbrock, R. Dengler and M. Leuwer. (2002). “Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol”. European Journal of Anaesthesiology 19 (8): 571–579. doi:10.1017/S0265021502000923.
- Brain KR, Green DM, Dykes PJ, Marks R, Bola TS; Green; Dykes; Marks; Bola (2006). “The role of menthol in skin penetration from topical formulations of ibuprofen 5% in vivo”. Skin Pharmacol Physiol 19 (1): 17–21. doi:10.1159/000089139. PMID 16247245.
- PDR for Herbal Medicines (4th ed.). Thomson Healthcare. p. 640. ISBN 978-1-56363-678-3.
- Croteau, R. B.; Davis, E.M.; Ringer, K. L; Wildung, M. R. (December 2005). “(−)-Menthol biosynthesis and molecular genetics”. Naturwissenschaften 92 (12): 562–77.Bibcode:2005NW…..92..562C. doi:10.1007/s00114-005-0055-0. PMID 16292524.
- Charles Sell (ed.). The Chemistry of Fragrances: From Perfumer to Consumer. ISBN 978-085404-824-3.
- Japan: Takasago to Expand L-Menthol Production in Iwata Plant
- After the company Haarmann & Reimer , now part of Symrise
- Schäfer, Bernd (2013). “Menthol”. Chemie in unserer Zeit 47 (3): 174. doi:10.1002/ciuz.201300599.














Sorry, the comment form is closed at this time.