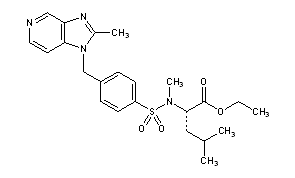
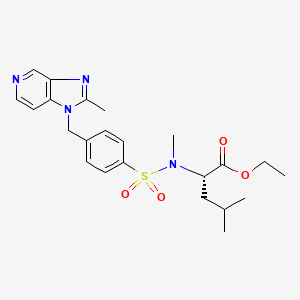
Lexipafant
CAS : 139133-26-9
N-Methyl-N-[[4-[(2-methyl-1H-imidazo[4,5-c]pyridin-1-yl)methyl]phenyl]sulfonyl]-L-leucine ethyl ester
N-methyl-N-[[a-(2-methyl-1H-imidazo[4,5-c]pyridin-1-yl)-p-tolyl]sulfonyl]-L-leucine ethyl ester
N-Methyl-N-[4-(2-methyl-1H-imidazo[4,5-c]pyridin-1-ylmethyl)phenylsulfonyl]-L-leucine ethyl ester
Manufacturers’ Codes: BB-882
DO6
GR-167089
ISV-611
GR-167089
ISV-611
UNII-H14917M9YW
Trademarks: Zacutex (Brit. Biotech)
MF: C23H30N4O4S
M Wt: 458.57
Percent Composition: C 60.24%, H 6.59%, N 12.22%, O 13.96%, S 6.99%
Properties: White crystalline solid from ethyl acetate, mp 105°. [a]D20 -6.7° (c = 2.0 in CDCl3).
Melting point: mp 105°
Optical Rotation: [a]D20 -6.7° (c = 2.0 in CDCl3)
Therap-Cat: Anti-inflammatory. (Nonsteroidal); Platelet Activating Factor Antagonist.
Lexipafant is a platelet-activating factor (PAF) antagonist that was in early clinical development at DevCo for the oral treatment of dementia and motor function disorders in HIV patients, intravenous treatment of acute pancreatitis, as well as for the prevention of certain serious renal and neurological complications experienced by patients undergoing cardiac surgery, including stroke. However, no recent developments of the drug candidate have been reported by the company.
Lexipafant was also being studied at British Biotech (now Vernalis) for the intravenous treatment of pancreatitis, but development for this indication was discontinued. In 2002, DevCo obtained from British Biotech exclusive rights to develop, manufacture and sell lexipafant for the treatment of human disease, excluding the fields of oncology and ophthalmology.
……………………………
……………………………………………
WO 1993016075
………………………………
WO 1995013064
Literature References:
Platelet activating factor (PAF) antagonist. Prepn: M. Whittaker, A. Miller, WO 9203422; eidem, US5200412 (1992, 1993 both to British Bio-Technology).
Structure-activity report: M. Whittaker et al., J. Lipid Mediators Cell Signalling 10, 151 (1994).
Pharmacology: F. M. Abu-Zidan et al., Pharmacol. Toxicol. 78, 23 (1996).
Clinical evaluation in acute pancreatitis: A. N. Kingsnorth et al., Br. J. Surg. 82, 1414 (1995).

Read all about Organic Spectroscopy on ORGANIC SPECTROSCOPY INTERNATIONAL 
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE













