
Zingerone Flower
Spice
In everyday life, ginger is used in cooking for its hot taste as well as its pungent smell.
|
Ecologists have studied the relationships of food and culture for many years. During this time, they have found surprising ties between spices that taste good and health-promoting side effects. An example of one of these spices is ginger. The sensory perception of ginger in the mouth and the nose arises from two distinct groups of chemicals:
|
|
|
|
Medicine
Ginger for many years has been the traditional remedy for colds. In modern medicine today, zingerone is used to treat a variety of medical problems. Zingerone reacts with free radicals that can cause tissue damage and inflammation. At Case Western University, research has been done showing that a topically applied extract containing zingerone may help prevent some skin cancers. In capsule form, ginger can also be used to replace anti-inflammatory drugs. In a recent study, ginger was found to be more effective than drugs in the treatment of nausea and motion sickness. Zingerone also has a major role in lipid oxidation since it is an anti-oxidant. It weakly inhibits oxidation of phospholipid liposomes in the presence of iron (III) and ascorbate to prevent heart-attacks.
 |
|
Zingerone used in pharmaceuticals |
It is these properties that have made zingerone a molecule of great importance and one that has been produced and synthesized for pharmaceutical use.
Structure
| 4-(4-hydroxy-3-methoxy-phenyl)-butan-2-one | 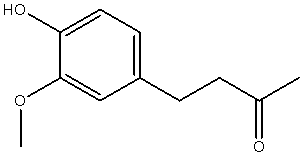 |
|
| Chemical Name | Structure of Zingerone | 3D Structure of Zingerone |
Physical Properties
| Synonyms | Zingerone; Vanillyl Acetone |
| Odour Description | Sweet, Spicy, Ginger, Vanilla, Woody |
| Appearance | Yellow To Yellow-Brown Crystals |
| Mol./Wt. | 194.2 |
| Formula | |
| Cas. # | 122-48-5 |
| Refractive Index | 1.54400 – 1.54500 @ 20.00 �C. |
| Melting Point | 40 – 41�C (Solvent – Aq. Ethanol) |
| Boiling Point | 141 �C. @ 0.5 Torr |
| Boiling Point | 290�C. @ 760.00 mmHg |
| Soluble in | Ethyl Alcohol, 1:1 In 50% Alcohol |
| Natural Occurrence | Ginger Root; Raspberry; Zingiber Officinale |
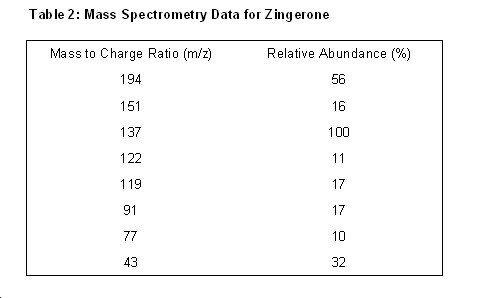
Mass Spectrum of Zingerone
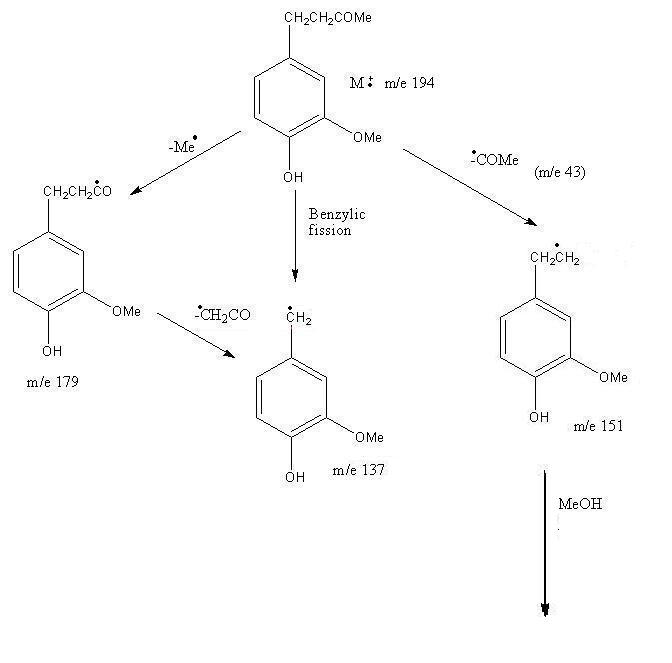
Pattern for Mass Fragmentation in the Spectrum
Infrared Spectrum of Zingerone
Table 3 : Infra Red spectral data on Zingerone

NOMURA METHOD
The Bunce & Reeves Method
Historical
Zingerone has been extracted from ginger for the past two thousand years.
A Brief History
| Common Name: | Ginger |
||||||||
| Latin Name: | Zingiber Officinale | ||||||||
| Family: | zingiberaceae | ||||||||
| Other Names: | Based on its origin:
|
The word ginger comes from the ancient Sanskrit ‘singabera’, meaning ‘shaped like a horn’. It first appeared in the writings of Confucius in the 5th century BC. and it has been used medicinally in the West for the past 2000 years. Various virtues have been ascribed to the spice; e.g. Henry VIII recommended it as a pro-phylactic against the plague. It was introduced by the Spaniards to the Americas and is now cultivated extensively in the West Indies. The Portuguese introduced it to West Africa. It is now used all over the world.
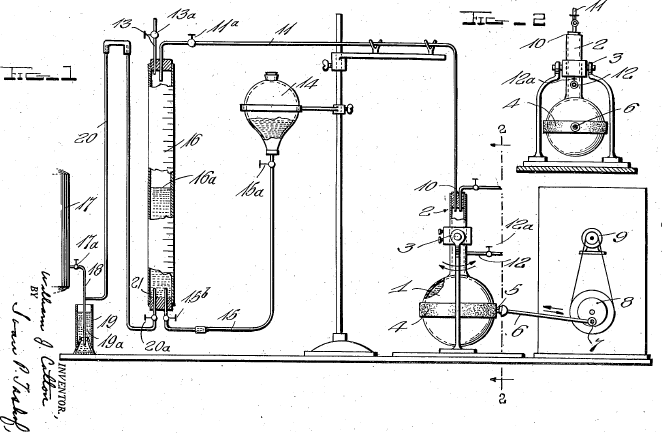
Only in the past century has zingerone been produced synthetically. A few interesting and unique syntheses have been chosen. Down the years, the technique of synthesis evolved. Though Lapworth & Wykes were the pioneers in the synthesis of zingerone, their method and reagents were not repeated. Below is a diagram of the apparatus used in their experiment.
Nomura, then Mannich and Merz founded the method used today. This is shown by Kim and Kim’s work. The other interesting method was formed by Bunce and Reeves. This method and the common one used today are elaborated on in the preparation section.
|
Year |
Method | Reagents |
| 1917 | Reduction and Decarboxylation of ethyl vanillylideneacetoacetateLapworth & Wykes | The Pioneers in the synthesis.Na Amalgam and NaOH solution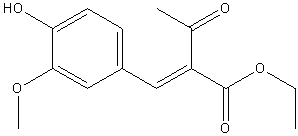
|
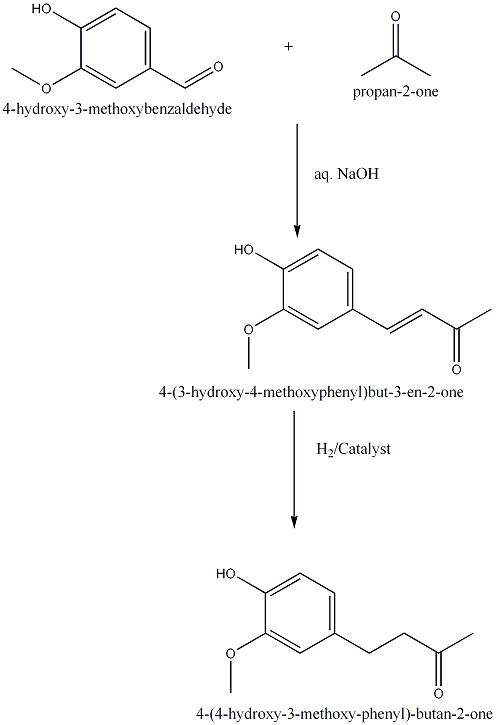
| 1925 | Reduction of 4-(4-hydroxy-3-methoxyphenyl)buten-2-one to 4-(4-hydroxy-3-methoxyphenyl)butan-2-one.Nomura | Na Amalgam and Water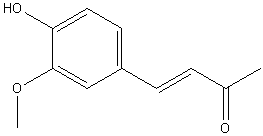 |
| 1927 | Reduction of 4-(4-hydroxy-3-methoxyphenyl)buten-2-one to 4-(4-hydroxy-3-methoxyphenyl)butan-2-one.Mannich & Merz | Pd Catalyst, |
| 1979 | Reaction of 4-benzyloxy-3-methoxybromomethylbenzene with the anion of acetone dimethylhydrazone, followed by oxidative hydrolysis, then hydrogenolysis to remove the benzyl groupEnders D. et Al | Pd/C, Hydrogen, Methanol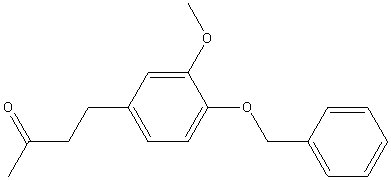 |
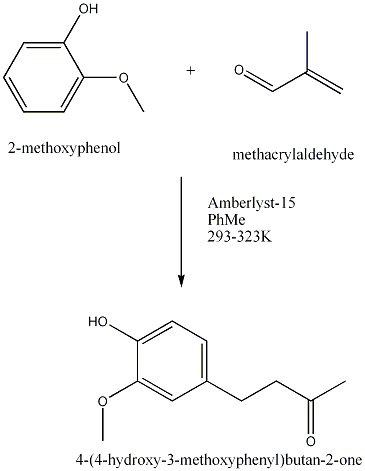
| 1989 | Amberlyst-15-catalyzed addition of 3-buten-2-one to the phenolBunce & Reeves | Amberlyst-15, Toluene, 20-50�C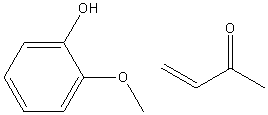 |
| 2004 | Reduction of 4-(4-hydroxy-3-methoxyphenyl)buten-2-one to 4-(4-hydroxy-3-methoxyphenyl)butan-2-one.Kim D. & Kim J. Y. | Pd/C Catalyst, |
Spice
 |
| Ginger |
As commented upon in the introduction, zingerone puts the ‘zing’ in ginger. In mustard oil, zingerone uses its properties to give it its flavour.
Zingerone has properties that give its strength of flavour. The higher molecular weight of zingerone in combination with the polar side-chain carbonyl group make zingerone molecules attract each other strongly. As a result, zingerone is not that volatile. The odour of ginger isn’t strong, but the hydrocarbon tail gives it a more intense flavour when it does come into contact with its receptor. Zingerone is also used in artificial flavouring and in fragrances.
A company called “Aroma Fragrance Fine Chemicals” (AFFC) have formulations are used globally for imparting attractive taste and aroma to processed foods and beverages. These chemicals can also add pleasing scents to perfumes, toiletries and detergents. Zingerone is one of their formulations. It is both a flavour and fragrance ingredient. This therefore means that zingerone is important in both processed foods and perfumes.
In industry, zingerone is therefore synthesised using the Kim & Kim’s method for this purpose.
Zingerone contains vanilloid (3-methoxy-4-hydroxy benzene) group in its structure. These phenolic hydroxyl groups provide the possibility to introduce a 4-ether-linked propanolamine side chain. Propanolamine derivatives were obtained by reacting zingerone with epichlorohydrin, and the obtained epoxide compounds were then reacted with isopropylamine, tert-butylamine or guaiacoxyethylamine, respectively, to yield 3 new derivatives shown below.
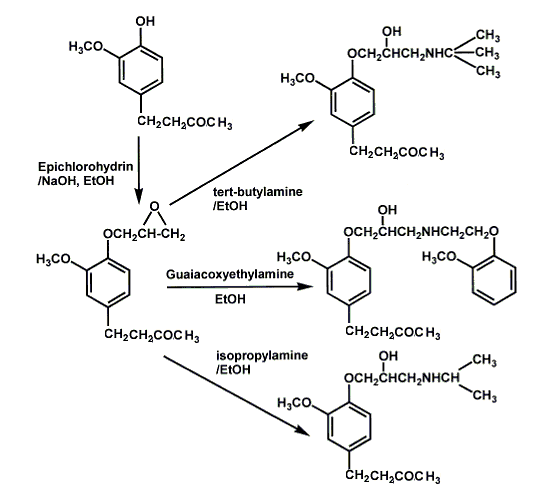
The syntheses above create third-generation β-adrenoceptor blockers, which possess ancillary cardiovascular actions other than β-adrenoceptor blockade, or cardioselective β-adrenoceptor blockers, have been shown to improve left ventricular function and decrease the risk of chronic heart failure. These β-adrenoceptor blockers are a break through in science and make the synthesis of zingerone very important in industry.
- Zingerone
- Ginger : www.wrc.net/wrcnet_content/herbalresources/materiamedica/materiamedica.aspx?mmid=13
- Introduction
- Picture of Bulbophyllum patens : Journal; Tan, Keng-Hong; Nishida, Ritsuo; JCECD8; J. Chem. Ecol.; EN; 26; 2; 2000; 533 – 546.
- Ginger Flower:http://www-ang.kfunigraz.ac.at/~katzer/engl/generic_frame.html?Zing_off.html
- Ecological Formulas Bottle : www.amazon.com
- Historical
- Cotton Diagram
Patent Number: US2381210
Publication date: 1945-08-07
Inventor(s): COTTON WILLIAM J
Applicant(s): PENNSYLVANIA COAL PRODUCTS COM
Requested Patent: US2381210
Application Number: US19430499408 19430820
Priority Number(s): US19430499408 19430820
IPC Classification:
EC Classification: C07C45/62
Equivalents:
- Cotton Diagram
- Characterisation
- NMR: Journal; Tan, Keng-Hong; Nishida, Ritsuo; JCECD8; J. Chem. Ecol.; EN; 26; 2; 2000; 533 – 546.
- IR Spectrum and Mass Spectrum: NIST Mass Spec Data Center, S.E. Stein, director (http://webbook.nist.gov)
- Mass Fragmentation: Journal; Locksley,H.D. et al.; JCPRB4; J. Chem. Soc. Perkin Trans. 1; EN; 1972; 3001-3006
- IR Bands: Journal; Das, B.; Takhi, M.; Kumar, H. M. Sampath; Srinivas, K. V. N. S.; Yadav, J. S.; PYTCAS; Phytochemistry; EN; 33; 3; 1993; 697-700.
- Physical Properties: http://www.thegoodscentscompany.com/data/rw1006231.html
- Spice
- http://www.ulg.ac.be/lcfi/licence/interros/interro10.html
- Medicine
- Molecule: http://mcb.berkeley.edu/labs/berger/Structure%20images/Collaboration/lr5lab.jpeg
- Reactions: Journal; Yeun-Chih Huang et al., Bioorganic & Medicinal Chemistry 9 (2001) 1739�1746
- Graph and Table: Journal; Pearson, D. A. et al.; J. Agric. Food Chem. 1997, 45, 578-582
- Diagram: http://images.medscape.com/pi/editorial/clinupdates/2002/2009/art-2009-1.fig16.gif
- Phytochemistry
- GC Diagram: Journal; Tan, Keng-Hong; Nishida, Ritsuo; JCECD8; J. Chem. Ecol.; EN; 26; 2; 2000; 533 – 546.
- Bulbophyllum patens (Phytochemistry): http://www.ulg.ac.be/lcfi/licence/interros/interro10.html











