MOM
 DIISOPROPYLAMINE
DIISOPROPYLAMINE
C6H15N
MW 101
the degree of unsaturation: the answer is 0. The molecule has no double bonds or rings.
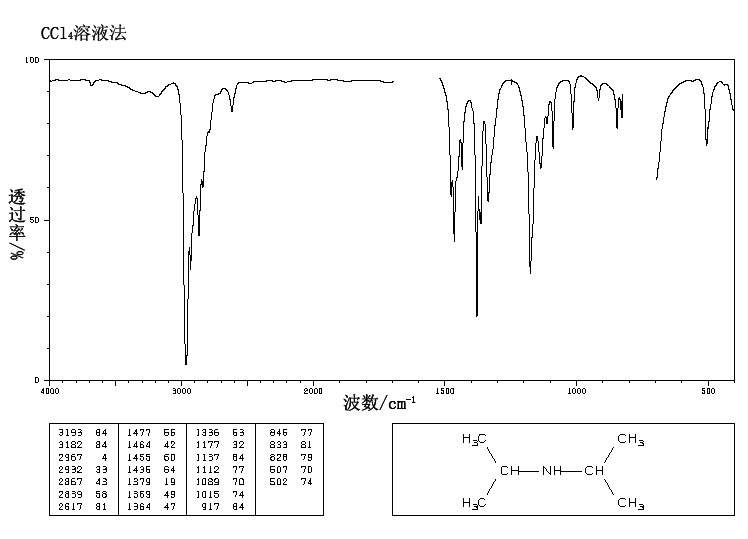
IR Spectrum
Since the molecule has a nitrogen, look for a band in the region 3400-3250 – there is a single small band at 3384, which probably indicates the N-H stretch of a secondary amine. (Recall that tertiary amines will not show a band in this region because they do not have any N-H’s to stretch.)

NMR Spectrum
Diisopropylamine(108-18-9)1HNMR
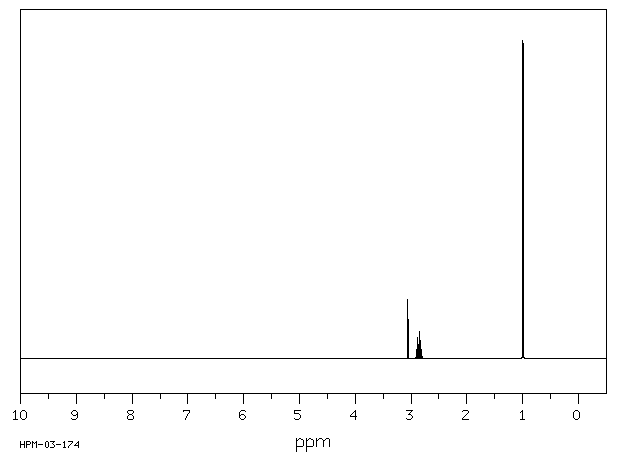

Amine protons show up from 0.5-3.0 ppm if the amine is not on an aromatic ring; the small “buried” peak at 1 ppm indicates a secondary amine peak:
![]()
There are only two other types of protons in the molecule: the doublet at 1 ppm indicates 12 hydrogens adjacent to one hydrogen and the septet at 2.9 ppm indicates 2 hydrogens adjacent to 6 hydrogens. The only way the molecule can be “put together” is to have each R group coming off the nitrogen to be the same, and to be -CH(CH3)2.


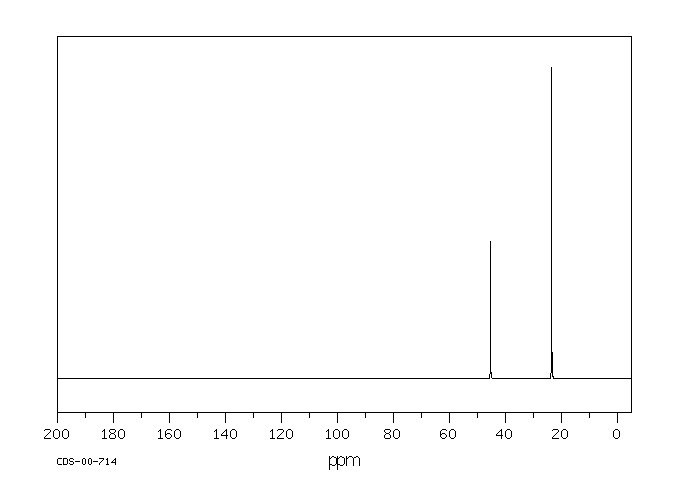
13C NMR
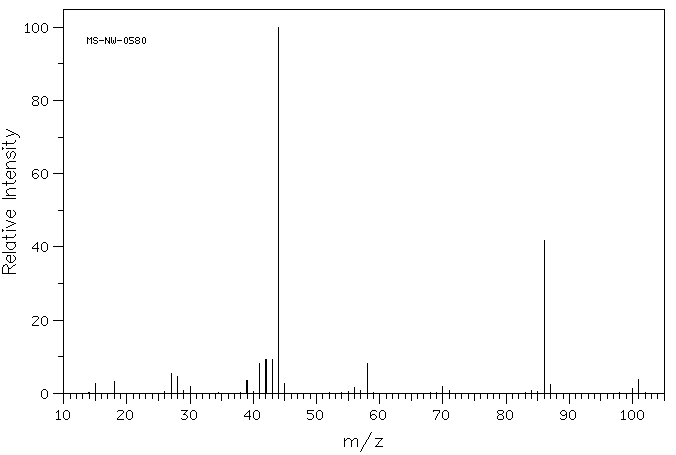
MASS
Summary
Example is diisopropylamine:















Sorry, the comment form is closed at this time.