
Lanoconazole
READ AT http://newdrugapprovals.org/2014/10/02/lanoconazole/
- Latoconazole, Lanoconazole, TJN-318, NND-318, Astat,
Nihon Nohyaku (Originator), Tsumura (Licensee)
| Synonym: | 2-[4-(2-Chlorophenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-yl-acetonitrile |
| Application: | An antifungal compound |
| CAS Number: | 101530-10-3 |
| Molecular Weight: | 319.83 |
| Molecular Formula: | C14H10ClN3S2 |
Brief background information
| Appearance: | Crystalline |
| Physical State: | Solid |
| Solubility: | Soluble in chloroform, and methanol. Insoluble in water. |
| Storage: | Store at -20° C |
| Melting Point: | 129-132 °C |
| Boiling Point: | ~477.6 °C at 760 mmHg (Predicted) |
| Density: | ~1.4 g/cm3 (Predicted) |
| Refractive Index: | n20D 1.73 (Predicted) |
| pK Values: | pKb: 3.76 (Predicted) |
| WGK Germany: | 3 |
| RTECS: | NI3393500 |
| PubChem CID: | 3002820 |
| Merck Index: | 14: 5357 |
| MDL Number: | MFCD00865590 |
| Beilstein Registry: | 4819111 |
| Salt | ATC | Formula | MM | CAS |
|---|---|---|---|---|
| – | D01 | C 14 H 10 ClN 3 S 2 | 319.84 g / mol | 101530-10-3 |

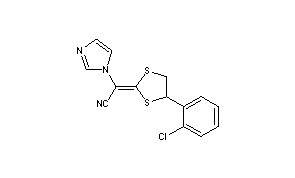
Application
-
antifungal
Synthesis pathway
| Synthesis a) |
|---|
  |
Trade Names
| Country | Trade name | Manufacturer |
|---|---|---|
| Japan | Astatine | Tsumura |
| Ukraine | No | No |
Formulations
-
1% cream;
-
1% ointment;
-
1% solution
Links
-
EP 218 736 (Nihon Nohyaku; EP-prior. 9.10.1985).
1. Oka, H., et al., 1992. Therapeutic efficacy of latoconazole in formulations of clinical use on experimental dermatophytosis in guinea pigs. Arzneimittel-Forschung. 42(3): 345-9. PMID: 1497697
2. Niwano, Y., et al., 1994. Therapeutic efficacy of lanoconazole, a new imidazole antimycotic agent, for experimental cutaneous candidiasis in guinea pigs. Antimicrobial agents and chemotherapy. 38(9): 2204-6. PMID: 7811048
3 http://aac.asm.org/content/38/9/2204.full.pdf
References 1. Seo, A., Kanno, H., Hasegawa, N. et al. (Nihon Nohyaku Co., Ltd.). Antimycotic agent and fungicidal agent. US 4738976. 2. Seo, A ., Sugano, H., Hasegawa, C., Ikeda, K., Munechica, Y., Konoe, T., Konaka, M. (Nihon Nohyaku Co., Ltd.). Antifungal agent. JP 87093227. 3. Seo , A., Sugano, H., Hasegawa, C., Ikeda, K., Nishimura, A., Miyashiro, Y. (Nihon Nohyaku Co., Ltd.). Non-medicinal bactericidal agents and method for their preparation. JP 87093204. 4. Seo, A., Sugano, H., Hasegawa, C., Miyashiro, Y., Nishimura, A., Ikeda, K. (Nihon Nohyaku Co., Ltd.). Ketene S, S-acetals. JP 85218387. 5. Seo, A., Kanno, H., Hasegawa, N. et al. (Nihon Nohyaku Co., Ltd.). A novel ketene S, S-acetal deriv., a process for manufacturing thereof and a method for curing mycosis by administering it. EP 218736.
MAKE IN INDIA
http://makeinindia.com/sector/pharmaceuticals/
Read all about Organic Spectroscopy on ORGANIC SPECTROSCOPY INTERNATIONAL 


One Response to “LANOCONAZOLE”
Sorry, the comment form is closed at this time.















Oh my goodness! Awesome article dude! Thank you so much, However I am having
issues with your RSS. I don’t understand the reason why I am unable to join it.
Is there anyone else having identical RSS problems? Anybody who knows the solution will you kindly respond?
Thanx!!