Example
C9H10O2
MW 150
Calculate the degree of unsaturation: the answer is 5. If the degree of unsaturation is 4 or greater, look for an aromatic ring, which has a degree of unsaturation of 4 (3 double bonds plus 1 ring). In addition to an aromatic ring, the molecule can have a carbon-carbon double bond, a carbonyl, or another ring.
IR Spectrum
Look for a carbonyl, since the degree of unsaturation indicates that the compound could have a double bond and we know that the molecule has an oxygen. There is a band at 1697, suggesting an alpha, beta unsaturated aldehyde or ketone(1710-1665). To see if the compound might be an aldehyde, look for bands in the region 2830-2695. In the spectrum below, note the two bands in this region, suggesting that the compound is indeed an aldehyde.
The IR can also help determine whether or not the compound is an aromatic (although the NMR is a better diagnostic method for this). Look for the C–H stretch in aromatics from 3100-3000. There are a couple very small bands in this region.
There is one more oxygen in the molecule, it could be an ether or even an ester (if we are incorrect in assuming an aldehyde is indicated). Ether IR bands are difficult to distinguish from any other C-O stretch band – the C-O stretch of alcohols, carboxylic acids, esters, and ethers all show up in the region 1320-1000 (see Ethers).
Consult the section on Aromatics for more information on IR spectroscopy of aromatics.

NMR Spectrum
From the IR, we know that compound is probably an aromatic and an aldehyde. Aldehydes and aromatics are quite distinctive in the NMR: aldehydes show up from 9-10, usually as a small singlet; aromatic protons show up from 6.5-8.5 ppm. Let’s look at the NMR:


The singlet at 9.9 ppm indicates an aldehyde; the 4 protons from 7-8 ppm indicate a di-substituted aromatic ring.
The remaining two peaks represent an ethyl group, -CH2CH3: 2 protons split by 3 protons adjacent to 3 protons split by 2 protons. The -CH2– portion of the ethyl group is shifted downfield further than the benzylic protons this indicates that it is next to an oxygen
Let’s draw an 8-carbon 10-hydrogen molecule incorporating these features: an aldehyde, a di-substituted aromatic ring, and an ethyl group:

The molecule is drawn as the para-substituted aromatic; this is indicated by the symmetry of the peaks in the aromatic region.
The following structure with the hydrogens in different colors shows how the protons correlate with the NMR peaks:


NOTE TWO AROM -H ORTHO TO O ATOM APPEAR AT 6.9 PPM
Summary
Example is 4-ethoxybenzaldehyde:

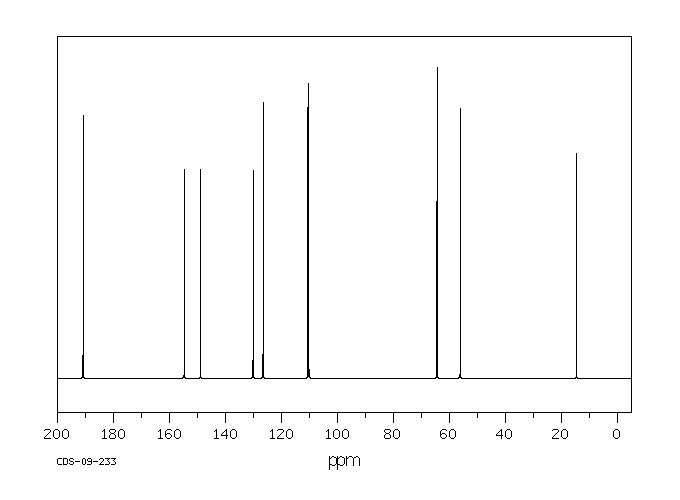
13 C NMR OF 3-Ethoxy-4-methoxybenzaldehyde(1131-52-8)
Alittle complicated than above example
The interpretation is available in form of numbering
13CNMR