Cerecor’s selective NMDA receptor subunit 2B antagonist CERC-301 (MK-0657) for depression in phase 2

CERC-301 (MK-0657) MK-657, c-6161, AGN-PC-00887R
structure source….http://www.google.com/patents/WO2013156614A1?cl=en my id is amcrasto@gmail.com
Treat depression; Treat major depressive disorder (MDD); Treat suicidality
808732-98-1 free form, C19 H23 F N4 O2
(-) (3S,4R) – 1-Piperidinecarboxylic acid, 3-fluoro-4-[(2-pyrimidinylamino)methyl]-, (4-methylphenyl)methyl ester,
AND
- 1-Piperidinecarboxylic acid, 3-fluoro-4-[(2-pyrimidinylamino)methyl]-, (4-methylphenyl)methyl ester, (3S,4R)-
- (-)-(3S,4R)-4-Methylbenzyl 3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate
- (3S,4R)-4-methylbenzyl 3-fluor-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate
- cas no of hydrochloride 808733-06-4
-
PLEASE NOTE THE + FORM
(+)-(3R,4S)-4-Methylbenzyl 3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate HAS CAS NO…..808732-99-2 AND ITS HYDROCHLORIDE 808733-07-5
also NOTE
- 1-Piperidinecarboxylic acid, 3-fluoro-4-[(2-pyrimidinylamino)methyl]-, (4-methylphenyl)methyl ester, (3R,4S)-rel-;
- cis-4-Methylbenzyl 3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate
- HAS CAS NO 808733-05-3 AND DELETED CAS 1221592-28-4
MY email ID IS amcrasto@gmail.com
AGN-PC-00887R, (4-methylphenyl)methyl (3S,4R)-3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylateMolecular Formula: C19H23FN4O2 Molecular Weight: 358.409923
Cerecor is developing the selective NMDA receptor subunit 2B antagonist CERC-301 (MK-0657) for depression.
CERC-301 (formerly MK-0657) is an oral, selective NMDA receptor subunit 2B (NR2B) antagonist in phase II clinical trials as adjunctive treatment for major depressive disorder (MDD) at Cerecor.
The compound had been in early trials at the National Institute of Mental Health (NIMH) for the treatment of major depression and at Merck & Co. for the treatment of Parkinson’s disease; however, no recent development has been reported in either case.
In 2013, the product was acquired by Cerecor from Merck & Co. on a worldwide basis for development and commercialization.
A phase II trial began in November 2013 and later that month, the FDA granted fast track designation for major depressive disorder.

………………………………………………
wo 2004108705 or http://www.google.co.in/patents/EP1648882B1?cl=en
METHODS OF SYNTHESIS
EXAMPLES 1 AND 2EXAMPLE 1
(35,4R)-4-methylbenzyl 3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylateEXAMPLE 2
(3R,4S)-4-methylbenzyl 3-fluor-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate
Step 1
Preparation of 4-Methylbenzyl 4-oxopiperidine-1-carboxylate:
-
4-Methylbenzyl alcohol (37.6 g, 308 mmol) was added to a solution of 1,1′-carbonyldiimidazole (50.0 g, 308 mmol) in DMF at RT and stirred for 1 h. 4-Piperidone hydrate hydrochloride (commercially available from Sigma-Aldrich, 47.0 g, 308 mmol) was added, resulting in a reaction mixture that was then heated to 50°C and stirred for 15 h. The reaction mixture was diluted with EtOAc and washed with 0.1 M HCl, H2O (four times), and brine, dried over Na2SO4, filtered and concentrated. Purification by silica gel chromatography (step gradient elution: 10%, 25%, 50% EtOAc in hexanes) produced the title compound (42.4 g, 85% yield) as a clear oil.
1H NMR (400 MHz, CDCl3) δ 7.24 (d, 2 H), 7.15 (d, 2 H), 5.08 (s, 2 H), 3.79 (t, 4 H), 2.45 (br s, 4 H) 2.31 (s, 3 H) ppm;
HRMS (ES) m/z 248.1281 [(M+H)+; calcd for C14H18NO3: 248.1287];
Anal. C14H17NO3: C, 68.03; H, 7.05; N, 5.59. Found: C, 68.00; H, 6.93; N, 5.66.
Step 2Preparation of (±)-4-methylbenzyl 3-fluoro-4-oxopiperidine-1-carboxylate:
-
A solution of 4-methylbenzyl 4-oxopiperidine-1-carboxylate (21.2 g, 85.7 mmol) and diisopropylethylamine (71.3 mL, 428 mmol) in dichloromethane (425 mL) was cooled to 0 °C and stirred. TBSOTf (29.5 mL, 129 mmol) was added slowly, maintaining the internal temperature below 5 °C. Aqueous NaHCO3 (20 mL) was added and the layers were separated. The organic layer was washed with NaHCO3, H2O (two times), and brine, dried over Na2SO4, filtered and concentrated to give the crude TBS enol ether.
-
The crude TBS enol ether was dissolved in DMF (125 mL) at RT. Selectfluor® reagent (commercially available from Air Products and Chemicals, Inc., 30.4 g, 85.7 mmol) was added and the reaction mixture was stirred for 10 min. The reaction mixture was partitioned between EtOAc and H2O and the organic layer was washed with H2O (three times). The combined aqueous layers were extracted with EtOAc (two times) and the combined organics were dried over Na2SO4, filtered and concentrated. The entire reaction above was repeated and the resulting reaction products were combined to give the title compound (40 g), which was used in the next step without purification. NMR and mass spectral data suggest the ketone functionality in the product exists as a hydrate.
1H NMR (400 MHz, CDCl3) δ 7.24 (m, 2 H), 7.19 (m, 2 H), 5.18 (s, 2 H), 4.81 (br d, 1 H), 4.50(br d, 1 H), 4.23 (d, 1 H), 3.90 (m, 1 H), 3.60 (m, 1 H), 3.35 (t, 1 H), 2.58 (m, 2 H), 2.35 (s, 3 H) ppm;
HRMS (ES) m/z 284.1292 [(M+H)+; calcd for C14H18FNO4: 284.1293];
Anal. C14H18FNO4•1.2H2O: C, 58.61; H, 6.46; N, 4.88. Found: C, 58.28; H, 6.06; N, 4.72.
Step 3Preparation of:
(±)-4-methylbenzyl (E)-4-(2-ethoxy-2-oxoethylidene)-3-fluoropiperidine-1-carboxylate
- and
(±)-4-methylbenzyl (Z)-4-(2-ethoxy-2-oxoethylidene)-3-fluoropiperidine-1-carboxylate
-
To a solution of (±)-4-methylbenzyl 3-fluoro-4-oxopiperidine-1-carboxylate (40 g, 150 mmol) in toluene (200 mL) at RT was added (carbethoxymethylene)triphenylphosphorane (63.0 g, 181 mmol) and the reaction mixture stirred for 1 h. The reaction mixture was concentrated and purified by silica gel chromatography (gradient elution: 10% to 20% EtOAc in hexanes) to give the olefins (±)-4-methylbenzyl (E)-4-(2-ethoxy-2-oxoethylidene)-3-fluoropiperidine-1-carboxylate and (±)-4-methylbenzyl (Z)-4-(2-ethoxy-2-oxoethylidene)-3-fluoropiperidine-1-carboxylate (41.0 g, 78% yield, 3 steps) as a 3:1 E:Z mixture. This mixture was utilized directly in the next step. A small sample of the mixture was separated by silica gel chromatography for characterization purposes.
(±)-4-methylbenzyl (E)-4-(2-ethoxy-2-oxoethylidene)-3-fluoropiperidine-1-carboxylate: white solid, 1H NMR (400 MHz, CDCl3) δ 7.26 (d, 2 H), 7.17 (d, 2 H), 5.98 (s, 1 H), 5.11 (s, 2 H), 4.85 (m, 1 H), 4.18 (q, 2 H), 4.08 (br d, 1 H), 3.70 (m, 1 H), 3.55 (m, 1 H) 3.41 (m, 1 H), 3.33, (m, 1 H), 2.63 (br d, 1 H), 2.35 (s, 3 H), 1.29 (t, 3 H) ppm;
HRMS (ES) m/z 358.1420 [(M+Na)+; calcd for C18H22FNO4Na: 358.1425];
Anal. C18H22FNO4: C, 64.21; H, 6.58; N, 4.27. Found: C, 64.46; H, 6.61; N, 4.18. -
(±)-4-methylbenzyl (Z)-4-(2-ethoxy-2-oxoethylidene)-3-fluoropiperidine-1-carboxylate: white solid, 1H NMR (400 MHz, CDCl3) δ 7.24 (d, 2 H), 7.15 (d, 2 H), 6.41(m, 1 H), 5.82 (s, 1 H), 5.11 (d, 2 H), 4.61 (m, 1H), 4.38 (br d, 1 H), 4.16 (q, 2 H), 3.05-2.95 (m, 1 H), 2.9-2.75 (m, 2 H), 2.33 (s, 3 H), 2.13 (m, 1 H), 1.27 (t, 3 H) ppm;
HRMS (ES) m/z 358.1422 [(M+Na)+; calcd for C18H22FNO4Na: 358.1425].
Step 4:Preparation of:
(±)-cis 4-methylbenzyl 4-(2-ethoxy-2-oxoethyl)-3-fluoropiperidine-1-carboxylate
and
(±)-trans 4-methylbenzyl 4-(2-ethoxy-2-oxoethyl)-3-fluoropiperidine-1-carboxylate
-
[0081]To a solution of the olefin mixture from Step 3 (10.0 g, 29.8 mmol) in toluene (160 mL) and CH2Cl2 (120 mL) was added diphenylsilane (5.53 mL, 29.8 mmol) and (R)-BINAP (1.86 g, 2.98 mmol). Sodium t-butoxide (0.29 g, 2.98 mmol) and CuCl (0.30 g, 2.98 mmol) were then added, the reaction mixture was protected from light and stirred for 15 h. Additional portions of diphenylsilane (2.76 mL), NaOtBu (0.29 g) and CuCl (0.30 g) were added and the reaction mixture was stirred at RT for 24h. The mixture was then filtered through celite and concentrated. Purification on silica gel (step gradient elution: 5%, 10%, 15%, 25%, 30% EtOAc in hexanes) gave recovered starting materials (3.5 g, 35% yield), (±)-cis 4-methylbenzyl 4-(2-ethoxy-2-oxoethyl)-3-fluoropiperidine-1-carboxylate (5.0 g, 50% yield) and (±)-trans 4-methylbenzyl 4-(2-ethoxy-2-oxoethyl)-3-fluoropiperidine-1-carboxylate (1.2 g, 12% yield).
(±)-cis 4-methylbenzyl 4-(2-ethoxy-2-oxoethyl)-3-fluoropiperidine-1-carboxylate: clear oil, 1H NMR (400 MHz, CDCl3) δ 7.25 (d, 2 H), 7.15 (d, 2 H), 5.10 (s, 2 H), 4.80-4.20 (m, 3 H), 4.15 (q, 2 H), 3.10-2.73 (m, 2 H), 2.52 (dd, 1 H), 2.35 (s, 3 H), 2.30 (dd, 1 H), 2.10 (m, 1 H), 1.72-1.48 (m, 2 H), 1.29 (t, 3 H) ppm;
HRMS (ES) m/z 338.1689 [(M+H)+; calcd for C18H25FNO4: 338.1762]. -
(±)-trans 4-methylbenzyl 4-(2-ethoxy-2-oxoethyl)-3-fluoropiperidine-1-carboxylate: clear oil, 1H NMR (400 MHz, CDCl3) δ 7.24 (d, 2 H), 7.15 (d, 2 H), 5.08 (s, 2 H), 4.50-3.95 (m, 3 H), 4.15 (q, 2 H), 2.81 (br t, 2 H), 2.70 (br d, 1 H), 2.35 (s, 3 H), 2.17 (m, 2 H), 1.89 (br d, 1 H), 1.25 (m, 1 H), 1.22 (t, 3 H) ppm;
HRMS (ES) m/z 338.1699 [(M+H)+; calcd for C18H25FNO4: 338.1762].
Step 5Preparation of (±)-((cis)-3-fluoro-1-{[(4-methylbenzyl)oxy]carbonyl}piperidin-4-yl)acetic acid:
-
To a solution of (±)-cis 4-methylbenzyl 4-(2-ethoxy-2-oxoethyl)-3-fluoropiperidine-1-carboxylate (10.0 g, 29.6 mmol) in THF (50 mL) was added aqueous NaOH (1M, 50 mL). The reaction mixture was stirred at RT for 5 h and then diluted with EtOAc and 1M HCl. The layers were separated and the aqueous extracted with EtOAc twice. The combined organics were washed with brine, dried over Na2SO4, filtered and concentrated to give the title compound (9.1 g) as a white solid which was used in the next step without further purification.
1H NMR (400 MHz, CDCl3) δ 7.24 (d, 2 H), 7.15 (d, 2 H), 5.08 (s, 2 H), 4.79-4.16 (m, 3 H), 3.05-2.75 (m, 2 H), 2.59 (dd, 1 H), 2.36 (dd, 1 H), 2.31 (s, 3 H), 2.20-2.02 (m, 1 H), 1.60 (m, 2 H) ppm;
HRMS (ES) m/z 310.1457 [(M+H)+; calcd for C16H21FNO4: 310.1449].
Anal. C16H20FNO4•0.15 H2O: C, 62.13; H, 6.52; N, 4.53. Found: C, 61.55; H, 6.37; N, 4.41.
Step 6Preparation of (±)-cis-4-methylbenzyl 4-(aminomethyl)-3-fluoropiperidine-1-carboxylate:
-
To a suspension of crude acid (±)-((cis)-3-fluoro-1-{[(4-methylbenzyl)oxy]carbonyl}piperidin-4-yl)acetic acid (9.1 g, 29.4 mmol) in toluene (80 mL) was added triethylamine (10.2 mL, 73.5 mmol) and diphenylphosphoryl azide (9.52 mL, 44.1 mmol). The reaction mixture was heated to 70 °C and stirred for 20 min. A mixture of dioxane (80 mL) and 1 M NaOH (80 mL) was added and the reaction mixture was cooled to RT. The reaction mixture was concentrated to remove the dioxane and extracted with EtOAc three times, dried over Na2SO4, filtered and concentrated. The residue was suspended in CH2Cl2, stirred for 30 min, and the white preciptate filtered off. The filtrate was concentrated to give crude product (7.5 g) as a yellow oil, used directly in the next step. An analytical sample was purified by silica gel chromatography (gradient elution: CH2Cl2 to 80:20:2 CH2Cl2 : MeOH : NH4OH) for characterization:
1H NMR (400 MHz, CDCl3) δ 7.24 (d, 2 H), 7.15 (d, 2 H), 5.08 (s, 2 H), 4.90-4.18 (m, 3 H), 2.95-2.75 (m, 2 H), 2.79 (dd, 1 H), 2.70 (dd, 1 H), 2.35 (s, 3 H), 1.59 (m, 3 H) ppm;
HRMS (ES) m/z 281.1658 [(M+H)+; calcd for C15H22FN2O2: 281.1660].
Step 7
Preparation of:
(3S,4R)-4-methylbenzyl 3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate
and
(3R,4S)-4-methylbenzyl 3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate
-
Two sealed tubes were each charged with a mixture of crude (±)-cis-4-methylbenzyl 4-(aminomethyl)-3-fluoropiperidine-1-carboxylate (Step 6, 3.7 g, 13.2 mmol) and 2-chloropyrimidine (1.51 g, 13.2 mmol) in n-butanol/diisopropyl-ethylamine (1:1, 13 mL). The tubes were sealed and the mixtures heated to 140 °C and stirred for 2 h. After cooling to RT, the reaction mixtures were combined and diluted with EtOAc and sat NaHCO3. The layers were separated and the organic was washed with H2O and brine, dried over Na2SO4, filtered and concentrated. Purification by silica gel chromatography (gradient elution: 1:1 hexanes:EtOAc to EtOAc) gave racemic cis-4-methylbenzyl 3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate (6.9 g, 65% yield, 3 steps) as a white solid.
-
The enantiomers were separated by preparative HPLC on a ChiralPak AD column (5 cm x 50 cm, 20µM) with MeOH:MeCN (15:85, 150 mL/min) as eluant. The HCl salt of Example 1 was prepared by dissolving (3S,4R)-cis-4-methylbenzyl 3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate (6.9 g, 19.3 mmol) in iPrOH (100 mL) at 65 °C. A solution of HCl in iPrOH (1.608 M, 12.6 mL, 20.2 mmol) was added and the solution was slowly cooled to RT over 15 h. Et2O (25 mL) was added, the mixture stirred for 3h, cooled to 0 °C, stirred for 1h and filtered to give (3S,4R)-4-methylbenzyl 3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate hydrochloride as a white solid (7.0 g, 92% recovery).
-
The hydrochloride salt of (3R,4S)-4-methylbenzyl-3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate was prepared using a similar procedure.
(3S,4R)-4-methylbenzyl 3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate•HCl:
-
[α]D -36.4° (c 0.17, MeOH);
Melting Point 149-150 °C;
1H NMR (400 MHz, CD3OD) δ 8.58 (br s, 2 H), 7.21 (d, 2 H), 7.17 (d, 2 H), 6.99 (t, 1 H), 5.06 (s, 2 H), 4.79 (m, 1 H), 4.42 (t, 1 H), 4.21 (d, 1 H), 3.60 (dd, 1 H), 3.50 (dd, 1 H), 3.15-2.80 (m, 2 H), 2.30 (s, 3 H), 2.10 (m, 1 H), 1.61 (m, 2 H) ppm;
HRMS (ES) m/z 359.1879 [(M+H)+; calcd for C19H24FN4O2: 359.1878];
Anal. C19H23FN4O2•HCl•0.2 H2O: C, 57.27; H, 6.17; N, 14.06. Found: C, 57.22; H, 6.37; N, 14.16.
(3R,4S)-4-methylbenzyl 3-fluoro-4-[(pyrimidin-2-ylamino)methyl]piperidine-1-carboxylate •HCl:
-
[α]D +34.9° (c 0.18, MeOH);
Melting Point 149-150 °C;
1H NMR (400 MHz, CD3OD) δ 8.58 (br s, 2 H), 7.21 (d, 2 H), 7.17 (d, 2 H), 6.99 (t, 1 H), 5.06 (s, 2 H), 4.79 (m, 1 H), 4.42 (t, 1 H), 4.21 (d, 1 H), 3.60 (dd, 1 H), 3.50 (dd, 1 H), 3.15-2.80 (m, 2 H), 2.30 (s, 3 H), 2.10 (m, 1 H), 1.61 (m, 2 H) ppm;
HRMS (ES) m/z 359.1870 [(M+H)+; calcd for C19H24FN4O2: 359.1878].
Anal. C19H23FN4O2•HCl•0.5H2O: C, 56.50; H, 6.24; N, 13.87. Found: C, 56.68; H, 6.27; N, 13.80.
……………….
WO 2006069287
http://www.google.com/patents/WO2006069287A1?cl=en
Scheme 1:
,
4-MeBnOH CDI
Scheme 2:
R1 X- R1
X” Rhodium metal precursor/
H I iiR2 chiral phosphine ligand |_] p — R:
14 13
Representative Examples include:
EXAMPLE 1
Step A:
11 -‘ .OH
A 5 L round bottom flask was charged with THF (1.87 L, KF< 50 ppm) and cooling to -75 °C was begun. When the temperature of THF had reached < – 20 °C, n-BuLi (11 M in hex, 123 mL) was added over 15 minutes in order to keep the solution temperature below -10 “C. When the solution reached -35 °C, controlled addition of diisopropylamine (197 mL, KF < 50 ppm) over 15 minutes was carried out so the exotherm did not cause the solution temperature to exceed -16 °C. The solution was then allowed to continue to cool until it reached -75 “C. 3-Fluoropyridine (compound 1 from Scheme 1) (125 g, KF < 150 ppm) was then added neat to this solution via addition funnel while maintaining the batch temperature below -70 °C.
Neat DMF (168 mL, KF < 50 ppm) was then added to the batch over 1 hour maintaining the temperature < -70 °C. After confirming complete formation of the aldehyde, the reaction was warmed to 0 “C, and H2O (230 mL, 10 eq.) was added. NaBH4 (48.4 g) was then added in two portions over 5 minutes at 0 °C. Addition of concentrated HCl (6 M, 1.17 L) was completed in 1 hour at temperatures between 0- 25°C. The rection batch was then heated to 40 °C and kept at this temperature for 1 hour.
The reaction was then allowed to cool to room temperature. Then, to the aqueous layer 6 M NaOH (747 mL) was slowly added at 0-15 °C to adjust the pH to 12. Approximately 700 mL of H2O was added to dissolve any precipitate in the aqueous layer. The aqueous layer was then extracted with IPAc (1 x 1.275 L, 2 x 800 mL). The organic layer was treated with 20 wt. % Darco-G60 carbon (based on product assay) and the solution was heated to 40 °C for 1 hour followed by filtration over solka floe. After filtration the organic layer was solvent switched from IPAc to IPAc:heptane (15-20% v/v IPAc:heptane). The product crystallized as a white solid. This solution was then cooled to 0 °C for 30 minutes and filtered. An additional 250 mL of heptane was cooled to 0 °C and used to wash the wet cake. Typical Yield = 79% (128.5 g).
Step B:
To a 2 L flask under N2 atmosphere were charged compound 2 from Scheme 1 (50.0Ig), acetone (524 mL), and BnBr (50.0 mL). This homogenous solution was heated to reflux for ~ 12 h. The reaction mixture was cooled to room temperature and diluted with heptane (550 mL). The pyridinium salt (compound 3 from Scheme 1) was collected by filtration. The wet cake was then slurry washed at ambient temperature with 25% acetone/heptane (200 mL) and filtered. The wet cake was then dried under vacuum at ambient temperature exposed to the atmosphere, affording a slight-pinkish solid ca. 98% pure by 1 H NMR
Typical Yield – 93% (109.5 g)
Step C:
To a 2 L round bottom flask were charged compound 3 from Scheme 1 (100.30 g, 1.00 eq.) and methanol (960 mL). The homogenous solution was then cooled to 100C. The NaBH4 (19.10 g, 1.50 Eq) was added portion wise (using a solid addition funnel) while keeping the temperature < 0 0C. The batch was diluted with IPAc (1.0 L), followed by addition of 1 L 11.25 wt% brine. The resulting mixture was aged 15 min, then allowed to separate into two clear layers. The lower brine layer was removed. The organic stream was then washed with 500 mL 15wt% brine, then allowed to separate into two clear layers. The lower brine layer was removed. The batch was adjusted to roughly 1:1 MeOHrIPAc (c = 100 g/L) and then treated with 25 wt% Ecosorb C-941 at 50 0C in for ~ 2 h. This was then filtered through a plug of celite, while rinsing with 1 : 1 MeOH:IPAc (rinse was roughly 25% of total batch volume). The batch was then concentrated to a residue.
The batch was then dissolved in 5% MeOH in IPAc at ~ 100 g/L (~ 636 mL). The batch was warmed to 50 0C, followed by addition of a solution of 4M HCl in dioxane (1.10 eq)) slowly over ~ 1 h. At this point, the batch was seeded with a small spatula tip full of seed. After complete addition of the HCl solution, the batch was allowed to cool to room temperature slowly overnight. The solids were isolated by filtration. A slurry cake wash was then performed with 5% MeOH/IPAc (200 mL), followed by a displacement wash of 5% MeOH/IPAc (200 mL). The batch was then dried under vacuum at ambient temperature exposed to the atmosphere to afford compound 4 as a white solid (77% yield).
This material, 66.1O g of crude 4, was dissolved in 450 mL MeOH to which was added 450 mL IPAc. This mixture was treated with 25wt% Ecosorb C-941 (16.53 g) and heated to 50 0C for 2 h. The mixture was then filtered through a pad of celite, washing the Ecosorb C-941 with ~ 500 ml 25% MeOH in IPAc. The mixture was then solvent switched on a rotovap to roughly 10% MeOH in IPAc. During the solvent switch, after concentrating to roughly 60% of its original volume, a small spatula tip full of seed was introduced, causing instant crystal growth. This mixture was concentrated until the final volume was ~ 350 mL. The slurry was then isolated, using a slurry wash of- 200 mL 5% MeOH/IPAc. The solids were dried over night under vacuum, exposed to the atmosphere, affording 60.23 g of 4 (70% yield).
Typical Yield = 70% (60.2 g).
Step D:
In a N2 atmosphere glovebox, (R,R)-Walphos (SL-W003-1) (60.1 mg, commercially available from Solvias, Inc., Fort Lee, New Jersey 07024) and [(COD)RhCl]2 (20.3 mg) were dissolved in dichloromethane (3 mL, anhydrous, N2 degassed) and aged for 45 min at room temperature. Compound 4 from Scheme 1 (15.0 g) was charged to a 6 oz. glass pressure vessel (Andrews Glass Co., Vineland, NJ) containing a magnetic stir bar. MeOH (69 mL, anhydrous, N2 degassed) was added, followed by the catalyst solution and a dichloromethane (3 mL) rinse.
The reactor was degassed with H2 (40 psig) and immersed in a preheated 50 0C oil bath. After a few minutes, the vessel was further pressurized with H2 to 85 psig and allowed to age for 18.75 h. After this time, the vessel was vented and cooled to room temperature. HPLC analysis indicated >99% conversion of the vinyl fluoride. HPLC analysis indicated 99.3% ee.
The reaction mixture from above was concentrated in vacuo to a dark brown oil, which was then diluted with 50 mL EtOAc, to which was added 50 mL saturated NaHCO3 (aq). This biphasic mixture was stirred at room temperature for 30 min. This mixture was separated, the aqueous layer was extracted 3 x 10 mL EtOAc, then the combined organic layers were dried over Na2SO4 and concentrated in vacuo to a residue, which was purified by column chromatography (1 : 1 EtOAc:hexanes) to afford 9.45 g of free base compound 5 (74.4% isolated yield) as a pale yellow oil.
Typical Yield = 74% (9.5 g).
HC1 HN^>”F
To a 100 mL round bottom flask was charged the free base compound 5 from Example Scheme 1 , (1.00 eq), the Pd(OH)2/C (1.29g), MeOH (23 mL), and 6M HCl (3.89 mL, 1.00 eq.). This mixture was degassed three times, finally filling the vessel with H2 (1 atm, balloon pressure). The reaction was stirred at room temperature for 18 h. The mixture was filtered through a plug of Celite 521, rinsed with 50 mL MeOH, then concentrated to a residue. The residue was redissolved in ~ 150 mL 1 : 1 MeOH:IPAc, then refiltered through a sintered glass funnel to remove inorganics. Theis resulting solution was then solvent switched to roughly 10% MeOH in IPAc, during which spontaneous crystallization of compound 6 from Scheme 1 was observed. The solids were isolated by vacuum, washed twice with ~ 10 mL 10% MeOH in IPAc, then dried under vacuum over night, affording a pale white, crystalline solid.
Typical Yield = 81% (3.2 g).
JV,iV -Carbonyldiimidazole, 2.39 g (1.00 eq) was charged to a 50 mL round bottom flask, to which was added the DMF (19.7 ml). Then, the 4- methylbenzyl alcohol (1.80 g 1.00 eq) was added as a solid. This mixture was stirred for 15 min. at room temperature, during which an exotherm was noted (ΔT = +6.1 0C, 18.5 0C to 24.6 0C). The fluoroalcohol HCl salt 6, 2.50 g (1.00 eq) was then added as a solid to this mixture. This was heated to 50 0C for 1O h, and then allowed to cool to room temperature over night. The resulting mixture was diluted with 40 mL EtOAc. This mixture was washed 2 x 25 mL 3M HCl and separated, then 1 x 25 mL 15wt% brine and separated. This was extracted with 1 x 15 mL EtOAc and combined with the previous organic stream. The organic stream was concentrated to a residue and subjected to column chromatography eluting with a gradient (0% to 50% EtOAc in hexanes, TLCs developed in 50% EtOAc:hexanes, visualizing with UV and KMnO4), to afford 3.35 g of a clear colorless oil.
Typical Yield = 81 % (3.4 g).
Step G:
A solution of fluoro alcohol compound 7 from Scheme 1 (1.22 g) in CH3CN was cooled to -20 °C and Hunig’s base (2.2 equiv., 1.66 mL) was added. To this, Tf2O – (1.1 equiv., 0.81 mL) was slowly added while maintaining the internal temperature < -10°C. Aqueous NH4OH (15 equiv., 2.7 mL) was then added to the reaction mixture at low temperature (-20°C) and then warmed up to room temperature and aged for Ih. After completion, toluene (15 mL) and 10% NaOH (10 mL) were added and the layers separated. After extraction, the organic layer was washed with H2O (IO mL).
The toluene stream of the amine was dried (-400 μg/mL) and concentrated to 100 g/L. Methanol was then added to obtain an overall solvent composition of toluene/MeOH (95:5), followed by the slow addition of HCl (1.05 equiv, 1.12 ml) at 50 °C. The amine hydrochloride 8 from Scheme 1 crystallized immediately, and the reaction was aged 20 min. The light yellow salt was then filtered and washed with cold toluene (15 mL) to offer amine hydrochloride 8 in 82% as a white crystalline solid.
Into a 100-L round bottom flask were charged 1.67 kg amine HCl salt 8 from Scheme 1, 912.4 g chloropyrimidine, 4.6 L of diisopropylethyl amine and 25.78 L ethylene glycol. The resulting slurry gradually became a solution, which was degassed and stirred under a nitrogen atmosphere. The contents were heated to 100 ° C for 12 h. The heat was turned off and the reaction solution slowly cooled to room temperature, which resulted in the formation of a slurry. To the slurry was added 77.3 L water over 1 h period and the slurry was aged at room temperature for 3 h. The mixture was filtered and the cake was washed with additional 80 L. The wet cake was left under nitrogen to dry overnight. After drying, 1.90 kg of an off white solid was collected.
1.77 kg of the above solid was dissolved into 71 L EtOAc and treated with 531 g Darco G-60 carbon at room temperature for 3 h. Filtration through Solka Floe was followed by washing with 2 x 20 L EtOAc. A solvent switch to MeOH under reduced pressure resulted in a slurry, and the final MeOH volume was adjusted to 19 L. The slurry in MeOH was heated to ca. 60 °C. Gradually cooling to room temperature resulted in a slurry, to which 57 L GMP water was added over 1 h with cooling (exothermic mixing, temperature controlled below 30 “C). The mixture was aged at room temperature for 3 h and filtered to collect solid, the cake was washed with 30 L GMP water and left to dry under nitrogen. 1.55 kg dried product was collected. (89% yield).
Typical Yield = 89% (1.55 kg).
………….
European Journal of Medicinal Chemistry (2012), 53, 408-415
http://www.sciencedirect.com/science/article/pii/S0223523412002310
Two diastereoisomeric NR2B NMDA antagonists were radiolabelled with fluorine-18. ► The radiolabelling of 3-[18F]fluoro-1,4-substituted-piperidine pattern was achieved. ► In vitro study showed high specific and selective binding for NR2B NMDAR receptors. ► Bmax/Kd ratios and logD7.4 demonstrated appropriate properties for in vivo imaging.

………………………..
Organic & Biomolecular Chemistry (2012), 10(42), 8493-8500
http://pubs.rsc.org/en/content/articlelanding/2012/ob/c2ob26378e#!divAbstract
In order to develop a novel and useful building block for the development of radiotracers forpositron emission tomography (PET), we studied the radiolabelling of 1,4-disubstituted 3-[18F]fluoropiperidines. Indeed, 3-fluoropiperidine became a useful building block in medicinal chemistry for the pharmacomodulation of piperidine-containing compounds. The radiofluorination was studied on substituted piperidines with electron-donating and electron-withdrawing N-substituents. In the instance of electron-donating N-substituents such as benzylor butyl, configuration retention and satisfactory fluoride-18 incorporation yields up to 80% were observed. In the case of electron-withdrawing N-substituents leading to carbamate or amidefunctions, the incorporation yields depend on the 4-susbtitutent (2 to 63%). The radiolabelling of this building block was applied to the automated radiosynthesis of NR2B NMDA receptor antagonists and effected by a commercially available radiochemistry module. The in vivoevaluation of three radiotracers demonstrated minimal brain uptakes incompatible with the imaging of NR2B NMDA receptors in the living brain. Nevertheless, moderate radiometabolism was observed and, in particular, no radiodefluorination was observed which demonstrates the stability of the 3-position of the fluorine-18 atom. In conclusion, the 1,4-disubstituted 3-[18F]fluoropiperidine moiety could be of value in the development of other radiotracers for PET even if the evaluation of the NR2B NMDA receptor antagonists failed to demonstrate satisfactory properties for PET imaging of this receptor.
![Graphical abstract Graphical abstract: Radiolabelling of 1,4-disubstituted 3-[18F]fluoropiperidines and its application to new radiotracers for NR2B NMDA receptor visualization](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C2OB26378E)
…………………….
WO 2013156614
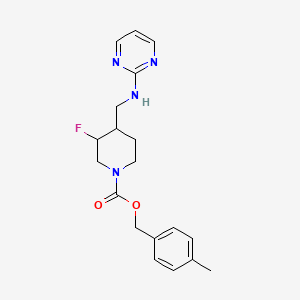
The chemical structure of MK-0657 is as follows
One Response to “Cerecor’s selective NMDA receptor subunit 2B antagonist CERC-301 (MK-0657) for depression in phase 2”
Sorry, the comment form is closed at this time.



































[…] http://www.allfordrugs.com/2014/04/17/cerecors-selective-nmda-receptor-subunit-2b-antagonist-cerc-30… […]