
Ranbezolid
392659-39-1 hydrochloride
392659-38-0 (free base)
N-{[(5S)-3-(3-Fluoro-4-{4-[(5-nitro-2-furyl)methyl]-1-piperazinyl}phenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl}acetamide
(S)-N-[[3-fluoro-4-[N-1[4-{2-furyl-(5-nitro)methyl}]piperazinyl]-phenyl]-2-oxo-5-oxazolidinyl]-methyl]acetamide


Infections due to Gram-positive bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecium (VRE), and penicillin-resistant Streptococcus pneumoniae(PRSP) are the leading cause of morbidity and mortality in hospital settings and community today. Oxazolidinones are a new class of totally synthetic antibacterial agents active against Gram-positive infections. Linezolid (Zyvox™, Pharmacia/Pfizer, is a drug in this class, approved in the United States and Europe for treatment of Gram-positive nosocomial and community-acquired pneumoniae and skin infections. Oxazolidinones inhibit the bacterial protein synthesis prior to the chain initiation step, by binding to the 23S rRNA of 50S ribosomal subunit, and interfering with the initiator fMet–tRNA binding to the P-site of the ribosomal peptidyltransferase centre

Ranbezolid hydrochloride, RBx-7644
|
9-23-2005
|
Plymorphic forms of phenyl oxazolidinone derivatives
|
The title compound is prepared by reductive alkylation of the known piperazinyl oxazolidinone derivative (I) with 5-nitro-2-furfural (II) in the presence of NaBH(OAc)3, followed by conversion to the corresponding hydrochloride salt.
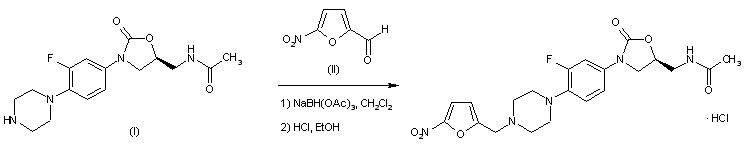
EP 1303511; US 2002103186; WO 0206278; WO 0307870; WO 0308389
…………….
synthesis
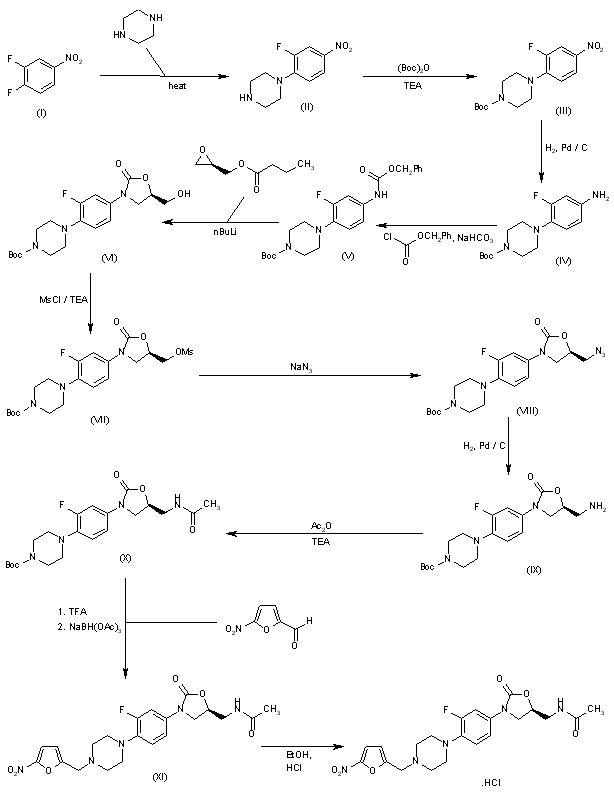
The antibacterial activity of RBx-7644 is due to the 5(S)-acetamidomethyl configuration at the oxazolidinone ring, and thus, asymmetric synthesis of only the 5(S)-enantiomer was desirable: 3,4-Difluoronitrobenzene (I) is condensed with piperazine in acetonitrile to give 4-(2-fluoro-4-nitrophenyl)-piperazine (II) as a light yellow compound. Compound (II) is dissolved in dichloromethane and triethylamine, followed by the addition of Boc-anhydride, to provide compound (III). 4-(tert-Butoxycarbonyl)-1-(2-fluoro-4-nitrophenyl)piperazine (III), upon hydrogenation with H2 over Pd/C in methanol at 50 psi, yields 4-(tert-butoxycarbonyl)-1-(2-fluoro-4-aminophenyl)piperazine (IV) as a dark solid. Compound (IV) reacts with benzylchloroformate in dry THF in the presence of solid sodium bicarbonate to afford the desired compound (V). 4-(tert-Butoxycarbonyl)-1-[2-fluoro-4-(benzyloxycarbonylamino)phenyl]piperazine (V), upon treatment with n-BuLi and (R)-glycidyl butyrate at -78 癈, gives the desired (R)-(-)-3-[3-fluoro-4-[4-(tert-butoxycarbonyl)piperazin-1-yl]phenyl]-5-(hydroxymethyl)-2-oxazolidinone (VI). The hydroxymethyl compound (VI) is treated with methanesulfonyl chloride in dichloromethane in the presence of triethylamine to give (R)-(-)-3-[3-fluoro-4-[4-(tert-butoxycarbonyl)piperazin-1-yl]phenyl]-5-(methylsulfonyloxymethyl)-2-oxazolidinone (VII). The sulfonyl derivative (VII) is treated with sodium azide in dimethylformamide to provide the azide (VIII) as a white solid. (R)-(-)-3-[3-Fluoro-4-[4-(tert-butoxycarbonyl)piperazin-1-yl)phenyl]-5-(azidomethyl)-2-oxazolidinone (VIII), upon hydrogenation with H2 over Pd/C at 45 psi, gives (S)-(-)-3-[3-fluoro-4-[4-(tert-butoxycarbonyl)-piperazin-1-yl]phenyl]-5-(aminomethyl)-2-oxazolidinone (IX). The aminomethyl compound (IX), upon treatment with acetic anhydride in dichloromethane in the presence of triethylamine, affords the acetamide derivative (X). The acetamidomethyl-oxazolidinone derivative (X), upon treatment with trifluoroacetic acid, gives (S)-(-)-3-[3-fluoro-4-(1-piperazinyl)phenyl]-5-(acetamidomethyl)-2-oxazolidinone, which, without isolation, is treated with 5-nitro-2-furaldehyde in the presence of sodium triacetoxy borohydride to provide compound (XI). Compound (XI), upon treatment with ethanolic HCl, affords RBx-7644 as a light yellow crystalline solid.
………………….

polymorphs
http://www.google.com/patents/US20050209248
(S)-N-[[3-fluoro-4-[N-1[4-{2-furyl-(5-nitro)methyl}]piperazinyl]-phenyl]-2-oxo-5-oxazolidinyl]-methyl]acetamidehydrochloride having the Formula I.
The compound of Formula I, namely, (S)-N-[[3-fluoro-4-[N-1 [4-{2-furyl-(5-nitro)methyl}] piperazinyl]-phenyl]-2-oxo-5-oxazolidinyl]-methyl]acetamide hydrochloride is a phenyl oxazolidinone derivative, as disclosed in PCT application WO 02/06278. It is said to be useful as antimicrobial agent, effective against a number of human and veterinary pathogens, including gram-positive aerobic bacteria, such as multiply resistant staphylococci, streptococci and enterococci as well as anaerobic organisms such as Bacterioides spp. andClostridia spp. species, and acid fast organisms such as Mycobacterium tuberculosis, Mycobacterium avium and Mycobacterium spp.
The PCT application WO 02/06278 describes the preparation of compounds of Formula I. The products of Formula I obtained by following the cited methods tend to be hygroscopic and difficult to filter. These types of disadvantageous properties have proven to be serious obstacles to the large-scale manufacture of a compound. Further, handling problems are encountered during the preparation of pharmaceutical compositions comprising the hygroscopic compound of Formula I obtained by following the method disclosed in WO 02/06278.
EXAMPLE 1 Preparation of Polymorphic ‘Form A’ of the Compound of Formula I
50 gm of free base of Formula I was dissolved in ethanol (750 ml) by heating at about 60° C. and to this solution was added ethanolic HCl (13.36 ml, 8.9 N) at about 45-50° C. The reaction mixture was cooled to about 10° C., and stirred for about 4 hours. The separated solid was filtered off and dried under vacuum at 60° C. The solid was then digested in ethanol (150 ml) at 70-80° C. for about 4 hours. It was then cooled to about 10° C., the solid was filtered and dried under vacuum at 60-65° C. to give 30 gm of the pure polymorphic ‘Form A’ of compound of Formula I.
………………

Synthesis and SAR of novel oxazolidinones: Discovery of ranbezolid
Bioorg Med Chem Lett 2005, 15(19): 4261
http://www.sciencedirect.com/science/article/pii/S0960894X05008310
Synthesis and SAR of novel oxazolidinones: Discovery of ranbezolidPages 4261-4267 |
||
Graphical abstract
Novel oxazolidinones were synthesized containing a number of substituted five-membered heterocycles attached to the ‘piperazinyl–phenyl–oxazolidinone’ core of eperezolid. Further, the piperazine ring of the core was replaced by other diamino-heterocycles. These modifications led to several compounds with potent activity against a spectrum of resistant and susceptible Gram-positive organisms, along with the identification of ranbezolid (RBx 7644) as a clinical candidate.
Substitution of five-membered heterocycles on to the ‘piperazinyl–phenyl–oxazolidinone’ core structure led to the identification of ranbezolid as a clinical candidate. Further replacement of piperazine ring with other diamino-heterocycles led to compounds with potent antibacterial activity.

Reagents and conditions: (a) Method A: TFA, CH2Cl2, 0 °C → rt; 5-chloromethyl-2-furaldehyde, potassium carbonate, DMF, rt; or (b) Method B: TFA, CH2Cl2, 0 °C → rt; 5-nitrofuran-2-carboxaldehyde, sodiumtriacetoxyborohydride, THF, molecular sieves 3 Å, rt. 7 = ranbezolid
-
Synthesis of compound 7: (S)-N-[[3-[3-Fluoro-4-(N-4-tert-butoxycarbonyl-piperazin-1-yl)phenyl]-2-oxo-5-oxa-zolidinyl]-methyl]acetamide (28a, 3.65 kg, 8.37 mol) was dissolved in dichloromethane (30.86 L) and cooled to 5 °C. To it trifluoroacetic acid (6.17 L) added dropwise and stirred for 14 h allowing the reaction mixture to warm to rt. The reaction mixture was evaporated in vacuo and the residue dissolved in tetrahydrofuran (58 L) followed by addition of molecular sieves 4 Å (4.2 kg). To the resulting mixture 5-nitro-2-furaldehyde (1.5 kg, 10.77 mol) was added followed by sodium triacetoxyborohydride (5.32 kg, 25.1 mol) and stirred for 14 h. The reaction mixture was filtered over Celite and filtrate evaporated in vacuo. The residue was dissolved in ethylacetate (85.6 L) and washed with satd sodium bicarbonate solution (36 L) and water (36 L). The organic layer was dried over anhyd sodium sulfate (3 kg) and evaporated in vacuo. The crude residue was purified by column chromatography (1–3% methanol in ethylacetate) to obtain (S)-N-[[3-[3-fluoro-4-[N-4-(5-nitro-2-furylmethyl)-piperazin-1-yl]phenyl]-2-oxo-5-oxa-zolidinyl]methyl]acetamide (39, 2.6 kg, yield 67%). Mp: 136 °C. 1H NMR (CDCl3): δ 7.42 (dd, 1H, phenyl–H), 7.29 (m, 2H, furyl–H), 7.07 (d, 1H, phenyl–H), 6.92 (t, 1H, phenyl–H), 6.51 (d, 1H, furyl–H), 6.11 (t, 1H, –NHCO–), 4.77 (m, 1H, oxazolidinone ring C5–H), 4.01 (t, 1H), 3.85–3.45 (m, 5H), 3.09 (m, 4H, piperazine–H), 2.72 (m, 4H, piperazine–H), 2.02 (s, 3H, –COCH3). MS m/z (rel. int.): 462.1 [(M+H)+, 100%], 484 [(M+Na)+, 25%], 500.2 [(M+K)+, 20%]. HPLC purity: 98%.
-
Compound 39(3.6 kg, 7.81 mol) was dissolved in abs ethanol (53.8 L) by heating to 60 °C. The resulting solution was cooled to 45 °C and ethanolic hydrochloride (1.48 L, 7.9 N) was added dropwise in 10 min. The mixture was then cooled to 10 °C and stirred for 4 h and the precipitate formed was filtered and washed with ethanol and dried to obtain (S)-N-[[3-[3-fluoro-4-[N-4-(5-nitro-2-furylmethyl)-piperazin-1-yl]phenyl]-2-oxo-5-oxazolidinyl]-methyl]acetamide hydrochloride, ranbezolid (7, 3.2 kg, yield from 39: 82%, yield from 28a: 55%).
- Ranbezolid
-
Mp: 207–209 °C.
-
1H NMR (DMSO, 300 MHz): δ 8.30 (t, 1H, –NHCO–), 7.75 (d, J = 3.3 Hz, 1H, furyl–H), 7.52 (dd, 1H, phenyl–H), 7.3–7.0 (m, 3H, phenyl–H, furyl–H), 4.70 (m, 1H, oxazolidinone ring C5–H), 4.63 (s, 2H), 4.08 (t, J = 8.8 Hz, 1H, –CH2–), 3.73 (t, J = 7.5 Hz, 1H), 3.43 (br m, piperazine–H merged with H2O in DMSO), 1.83 (s, 3H, –COCH3).
-
HPLC purity: 98%. Anal. Calcd for C21H25ClN5O6·0.5H2O: C, 50.76; H, 5.48; N, 14.09. Anal. Found: C, 50.83; H, 5.17; N, 13.83.
References
- European Journal of Pharmacology. 2006. 545, 167–172
- US2005209248, 9-23-2005
Plymorphic forms of phenyl oxazolidinone derivatives -
1-1-2013Anti-anaerobic potential of ranbezolid: insight into its mechanism of action against Bacteroides fragilis.International journal of antimicrobial agents11-15-2009Synthesis and biological activity of novel oxazolidinones.Bioorganic & medicinal chemistry letters4-1-2009Mode of action of Ranbezolid against staphylococci and structural modeling studies of its interaction with ribosomes.Antimicrobial agents and chemotherapy8-1-2008Effect of oxazolidinone, RBx 7644 (ranbezolid), on inhibition of staphylococcal adherence to plastic surfaces.Journal of chemotherapy (Florence, Italy)4-1-2008Utilization of Bombyx mori larvae as a surrogate animal model for evaluation of the anti-infective potential of oxazolidinones.Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy9-15-2007Synthesis and in vitro antibacterial activity of novel methylamino piperidinyl oxazolidinones.Bioorganic & medicinal chemistry letters9-18-2006Ranbezolid, a novel oxazolidinone antibacterial: in vivo characterisation of monoamine oxidase inhibitory potential in conscious rats.European journal of pharmacology10-1-2005Synthesis and SAR of novel oxazolidinones: discovery of ranbezolid.Bioorganic & medicinal chemistry letters6-1-2005Activity of RBx 7644 and RBx 8700, new investigational oxazolidinones, against Mycobacterium tuberculosis infected murine macrophages.International journal of antimicrobial agents10-1-2004In vitro activity of RBx 7644 (ranbezolid) on biofilm producing bacteria.International journal of antimicrobial agents
-
3-1-2003Antianaerobe activity of RBX 7644 (ranbezolid), a new oxazolidinone, compared with those of eight other agents.Antimicrobial agents and chemotherapy3-1-2003Antipneumococcal and antistaphylococcal activities of ranbezolid (RBX 7644), a new oxazolidinone, compared to those of other agents.Antimicrobial agents and chemotherapy















Sorry, the comment form is closed at this time.