
Surotomycin
http://www.ama-assn.org/resources/doc/usan/surotomycin.pdf
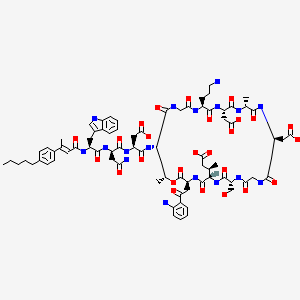
N-[(2E)-3-(4-Pentylphenyl)-2-butenoyl]-D-tryptophyl-D-asparaginyl-N-[(3S,6S,9R,15S,18R,21S,24S,30S,31R)-3-[2-(2-aminophenyl)-2-oxoethyl]-24-(3-aminopropyl)-15,21-bis(carboxymethyl)-6-[(2R)-1-carboxy-2 -propanyl]-9-(hydroxymethyl)-18,31-dimethyl-2,5,8,11,14,17,20,23,26,29-decaoxo-1-oxa-4,7,10,13,16,19,22,25,28-nonaazacyclohentriacontan-30-yl]-L-α-asparagine
MOLECULAR FORMULA C77H101N17O26
MOLECULAR WEIGHT 1680.7
SPONSOR Cubist Pharmaceuticals, Inc.
CODE DESIGNATION CB-183,315
CB-315, CB-183315, CB-183,315
CAS REGISTRY NUMBER 1233389-51-9
U.S. – Fast Track (Treat Clostridium difficile-associated diarrhea (CDAD));
U.S. – Qualified Infectious Disease Program (Treat Clostridium difficile-associated diarrhea (CDAD))
| Company | Cubist Pharmaceuticals Inc. |
| Description | Oral antibacterial lipopeptide |
| Therapeutic Modality | Macrocycle |
| Latest Stage of Development | Phase III |
| Standard Indication | Diarrhea (infectious) |
| Indication Details | Treat Clostridium difficile-associated diarrhea (CDAD) |
EMEA……..
| Name | |||
|---|---|---|---|
| P/0096/2013: EMA decision of 29 April 2013 on the agreement of apaediatric investigation plan and on the granting of a deferral for surotomycin (EMEA-001226-PIP01-11) |
Surotomycin is an investigational oral antibiotic. This antibiotic is under investigation for the treatment of life-threatening Diarrhea, commonly caused by the bacteria Clostridium difficile.[1]
CB-183315 is an investigational antibacterial drug candidate in phase III clinical trials at Cubist for the treatment of Clostridium difficile-associated diarrhea. It is a potent, oral, cidal lipopeptide. In 2012, Qualified Infectious Disease Product Designation was assigned in the U.S. for the treatment of clostridium difficile-associated diarrhea (CDAD).
Surotomycin (CB-315)
Surotomycin is an antibacterial lipopeptide discovered by Cubist scientists in our research laboratories in Lexington, Massachusetts. Surotomycin is both bactericidal against Clostridium difficile and more potent than vancomycin in vitro. Surotomycin stays at the site of infection in the bowel, with minimal systemic absorption and it does not interfere with normal bowel flora. Based on its features and its preclinical safety profile, Cubist filed an Investigational New Drug (IND) Application for surotomycin in December 2008.
Following safety and pharmacokinetic studies in healthy human volunteers, Cubist began a Phase 2 study in April 2010 to assess the safety and efficacy of surotomycin in patients with CDAD, in particular to assess its ability to reduce relapse rates. In this trial of 209 patients, two different doses of surotomycin were studied and compared with oral vancomycin. The higher dose demonstrated a high clinical cure rate as evidenced by resolution of diarrhea, comparable to oral vancomycin. The most interesting results in this study, however, relate to recurrence rates. The percent of patients who had an initial response to treatment but who subsequently had a recurrence or relapse was 36 percent in the oral vancomycin arm and was 17 percent in the surotomycin 250mg treatment group — about a 50% reduction in relapse rate, which was statistically significant. In this trial, 32% of patients were infected with the hypervirulent NAP-1 strain of C. difficile. The clinical response rate in the subset of patients infected with the NAP-1 strain was comparable across the surotomycin and oral vancomycin groups. Though not statistically significant, there was a modest reduction in the relapse rates in the subset of surotomycin patients infected with NAP-1 strains.
The ability to reduce relapses is important to both patients and health care providers. In the Phase 2 study we assessed the impact of surotomycin and oral vancomycin on normal bowel flora. Treatment with surotomycin had a very minimal impact on levels of Bacteroides, a key normal bowel bacterial species, compared to oral vancomycin which resulted in a marked depletion of stool levels of these bacteria during treatment. Why does this matter? The reason is — bowel flora like Bacteroides are critical in providing a competitive environment in the bowel that prevents C. difficile overgrowth. We believe that it is this difference in impact on normal bowel flora that helps explain the differences seen in recurrence rates following treatment with Surotomycin versus oral vancomycin.
Surotomycin’s Phase 3 program includes two identical global, randomized, double-blind, active-controlled, multi-center trials. The primary objective is to demonstrate non-inferiority of surotomycin versus the comparator, oral vancomycin, in clinical response at the end of treatment in adult subjects with CDAD, using a non-inferiority margin of 10%. We also have designed this trial to allow us to demonstrate that sustained clinical response to surotomycin at the end of the study is superior to oral vancomycin. Also, we will fully evaluate the safety of surotomycin in the study subjects.
In late 2012 Cubist received from the FDA a Qualified Infectious Disease Product (QIDP) designation for surotomycin. Additionally, in early 2013 Cubist was granted Fast track status for surotomycin. The QIDP designation and subsequent granting of Fast Track status was made possible by the GAIN Act, Title VIII (Sections 801 through 806) of the Food and Drug Administration Safety and Innovation Act. The GAIN Act provides pharmaceutical and biotechnology companies with incentives to develop new antibacterial and antifungal drugs for the treatment of life-threatening infectious diseases caused by drug resistant pathogens. Qualifying pathogens are defined by the GAIN Act to include multi-drug resistant Gram-negative bacteria, including Pseudomonas, Acinetobacter, Klebsiella, and Escherichia coli species; resistant Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus; multi-drug resistant tuberculosis; and Clostridium difficile.
About CDAD
CDAD is a disease caused by an overgrowth of, and subsequent toxin production by, C. difficile, a resident anaerobic spore-forming Gram-positive bacterium of the lower gastrointestinal tract. This overgrowth is caused by the use of antibiotics for the treatment of common community and hospital acquired infections (HAIs). Although they treat the underlying infection, many antibiotics disrupt the natural gut flora and allow C. difficile to proliferate. C. difficile produces enterotoxin and cytotoxin, which can lead to severe diarrhea, sepsis and even death. While most types of HAIs are declining, the infection caused by C. difficile remains at historically high levels. According to the latest data from the Centers for Disease Control, C. difficile continues to be the leading cause of death associated with gastroenteritis in the US. For CDAD alone, there was more than a five-fold increase in deaths between 1999 and 2007. C. difficile causes diarrhea linked to 14,000 American deaths each year. About 25% of C. difficile infections first show symptoms in hospital patients; 75% first show in nursing home patients or in people recently cared for in doctors’ offices and clinics. C. difficile infections cost at least $1 billion in extra health care costs annually.
SUROTOMYCIN
CB-183,315 is a cyclic lipopeptide antibiotic currently in Phase III clinical trials for the treatment of Clostridium difficile-associated disease (CDAD). As disclosed in International Patent Application WO 2010/075215, herein incorporated by reference in its entirety, CB-183,315 has antibacterial activity against a broad spectrum of bacteria, including drug-resistant bacteria and C. difficile. Further, the CB-183,315 exhibits bacteriacidal activity.
CB-183,315 (Figure 1) can be made by the deacylation of BOC-protected daptomycin, followed by acylation and deprotection as described in International Patent Application WO 2010/075215.
During the preparation and storage of CB-183,315, the CB-183,315 molecule can convert to structurally similar compounds as shown in Figures 2-4, leading to the formation of anhydro-CB-183,315 (Figure 3) and beta-isomer of CB-183,315 (“B- isomer CB183,315” in Figure 2). Accordingly, one measure of the chemical stability of CB- 183 ,315 is the amount of CB- 183 ,315 (Figure 1 ) present in the CB- 183 ,315 composition relative to the amount of structurally similar compounds including anhydro-CB-183,315 (Figure 3) and beta-isomer of CB-1 83,315 (Figure 2). The amount of CB-183,315 relative to the amount of these structurally similar compounds can be measured by high performance liquid chromatography (FIPLC) after reconstitution in an aqueous diluent (e.g., as described in Example 10). In particular, the purity of CB-183,315 and amounts of structurally similar compounds (e.g., Figures 2, 3 and 4) can be determined from peak areas obtained from HPLC (e.g., according to Example 10 herein), and measuring the rate of change in the amounts of CB-183,315 over time can provide a measure of CB-183,315 chemical stability in a solid form.
There is a need for solid CB-183,315 compositions with improved chemical stability in the solid form (i.e., higher total percent CB-183,315 purity over time), providing advantages of longer shelf life, increased tolerance for more varied storage conditions (e.g., higher temperature or humidity) and increased chemical stability.
……………..
WO2010075215A1
http://www.google.com/patents/WO2010075215A1?cl=en ………… copy paste link
Example 1
Preparation of N-{1 -[(E)-3-(4-pentylphenyl)but-2-enoyl]}-L-tryptophyl-D- asparaginyl-L-α-aspartyl-L-threonylglycyl-L-ornithyl-L-α-aspartyl-D-alanyl-L-α- aspartylglycyl-D-seryl-(3R)-3-methyl-L-α-glutamyl-(αS)-α,2-diamino-γ- oxobenzenebutanoic acid (13→4)-lactone (49).
1003 1004
Step 1 : Preparation of (E)-ethyl 3-(4-pentylphenyl) but-2-enoate (1002).
A mixture of commercially available 1-(4-pentylphenyl)ethanone (5 g, 26.3 mmol) and (ethoxycarbonylmethylene)-triphenylphosphorane (18.3 g, 52.5 mmol) was stirred at 150 0C for 48 hours under a nitrogen atmosphere. The reaction mixture was cooled to ambient temperature and diluted with ethyl acetate (50 ml_) and petroleum ether (200 ml_). The suspension was filtered through a fritted funnel. The concentrated filtrate was purified by flash column chromatography with silica gel (petroleum ether : ethyl acetate = 80:1 ) to give the title compound (1.6 g) having the following physical data: 1H NMR (300 MHz, δ, CDCI3) 0.90 (br, 3H), 1.36 (br, 7), 1.63 (br, 2H), 2.58 (s, 3H), 2.63 (br, 2H), 4.22 (q, 2H), 6.15 (s, 1 H), 7.20 (d, 2H), 7.41 (d, 2H).
Step 2: Preparation of (E)-3-(4-pentylphenyl) but-2-enoic acid (1003).
A solution of compound 1002 (1.5 g, 5.77 mmol) in ethanol (50 ml_) and 3N potassium hydroxide (25 ml_) was stirred at 45 0C for 3 hours. The reaction mixture was concentrated and the resulting residue was diluted with water (50 ml_). The aqueous solution was acidified to pH 2 with 1 N hydrochloric acid and extracted with EtOAc (2 * 30 ml_). The combined organic layers were dried with anhydrous sodium sulfate, filtered and concentrated. The residue was purified by flash column chromatography (silica gel, petroleum ether : ethyl acetate = 10:1) to afford the title compound (0.95 g) having the following physical data: 1 H NMR (300 MHz, δ, CDCI3) 0.90 (br, 3H), 1.33 (br, 4H), 1.62 (br, 2H), 2.60 (br, 5H), 6.18 (s, 1 H), 7.18 (d, 2H), 7.42 (d, 2H).
Step 3: Preparation of (E)-3-(4-pentylphenyl)but-2-enoyl chloride (1004).
Oxalyl chloride (3.2 mL, 36.60 mmol) and DMF (50 μl_) were added drop wise to a solution of compound 1003 (5.0 g, 21.52 mmol) in dichloromethane (100 mL) at 0 0C. The reaction solution was warmed up to room temperature and stirred for 4 hours. The reaction mixture was concentrated in vacuum and the residue was dried under hi-vacuum for 3 hours. The crude product was used in the next step without further purification.
Step 4: Preparation of N-{1 -[(E)-3-(4-pentylphenyl)but-2-enoyl]}-L-tryptophyl-D- asparaginyl-L-α-aspartyl-L-threonylglycyl-L-[(N-tert-butoxycarbonyl)-ornithyl]-L-α- aspartyl-D-alanyl-L-α-aspartylglycyl-D-seryl-(3R)-3-methyl-L-α-glutamyl-(αS)-α,2- diamino-γ-oxobenzenebutanoic acid (13-→4)-lactone (1005).
Deacylated BOC-protected daptomycin (3.5Og, 2.23 mmol) and sodium bicarbonate (1.13 g, 61.0 mmol) were dissolved in THF (130 mL) and water (50 mL). The deacylated BOC-protected daptomycin sodium bicarbonate solution was cooled to 0 0C. and a solution of compound 1004 (1.96 g, 7.82 mmol) in THF (20 mL) was then introduced. The reaction mixture was warmed to room temperature and stirred for 4 hours. The mixture was concentrated in vacuum to remove THF. The remaining aqueous solution was loaded on a C18 flash chromatography column (35mηnχ 300mm, Bondesil HF C18 resin purchased from Varian). The column was first washed with water to remove salt and then with methanol to wash out product. Crude compound 1005 (3.46 g) was afforded as a white solid after removal of methanol. MS m/z 1780.8 (M + H)+.
Steps 5-6: Preparation of N-{1-[(E)-3-(4-pentylphenyl)but-2-enoyl]}-L-tryptophyl- D-asparaginyl-L-α-aspartyl-L-threonylglycyl-L-ornithyl-L-α-aspartyl-D-alanyl-L-α- aspartylglycyl-D-seryl-(3R)-3-methyl-L-α-glutamyl-(αS)-α,2-diamino-γ- oxobenzenebutanoic acid (13→4)-lactone (49).
TFA (10 ml_) was added to a solution of compound 1005 (3.46 g) in DCM (50 mL) at room temperature. The reaction mixture was stirred vigorously for 45 minutes and added slowly to vigorously stirring diethyl ether (100 mL). The resulting yellow precipitation was collected by filtration. The crude product was purified by Preparative HPLC to afford the TFA salt of compound 6 (0.75 g). MP carbonate resin (purchased from Biotage) was added to the solution of compound 6 TFA salt (0.70 g, 0.39 mmol) in anhydrous methanol (30.0 mL). The mixture was stirred at room temperature for 4 hours. The resins were removed by filtration and rinsed with methanol. The methanol solution was concentrated under vacuum to give product as off-white solid (408 mg). MS m/z 1680.7 (M + H)+.
Example 1 b
Alternative preparation of N-{1-[(E)-3-(4-pentylphenyl)but-2-enoyl]}- L-tryptophyl-D-asparaginyl-L-α-aspartyl-L-threonylglycyl-L-ornithyl-L-α-aspartyl-D- alanyl-L-α-aspartylglycyl-D-seryl-(3R)-3-methyl-L-α-glutamyl-(αS)-α,2-diamino-γ- oxobenzenebutanoic acid (13→4)-lactone (49).
daptomycin,
1003
A solution of (E)-3-(4-pentylphenyl)but-2-enoic acid (1 100 g, 4.73 mol), Λ/-Ethyl-Λ/’-(3-dimethylaminopropyl)carbodiimide hydrochloride (907 g, 4.73 mol), HOBT (640 g, 4.73 mol) and 4-(dimethylamino)pyridine (22 g, 0.18 mol) in DMF (11 L) was stirred at room temperature for 4 hours at which point the activation of the (E)-3-(4-pentylphenyl)but-2-enoic acid was deemed complete by HPLC.
This reaction mixture was added to a suspension of Deacylated BOC- protected daptomycin (2600 g, 1.66 mol), sodium bicarbonate (804 g, 9.57 mol) in water (11.25 L) and 1 ,4-dioxane (33.75 L). The mixture was stirred at room temperature for 2.5 hours at which time HPLC indicated complete consumption of Deacylated BOC-protected daptomycin. The reaction mixture was diluted with water (22.5 L) and cooled with an ice bath. Concentrated hydrochloric acid (5.25 L) was added while maintaining the internal temperature below 30 0C. After the addition, the solution was stirred at room temperature for 5 days at which time HPLC indicated complete consumption of the Boc protected intermediate.
The reaction mixture was washed with methyl terf-butyl ether (90 L then approximately 60 L then approximately 45 L then approximately 45 L) to remove 1 ,4-dioxane. The remaining solution (approximately 44 L) was adjusted to pH 2.69 with 2N sodium hydroxide (11.3 L) and water (53.4 L). This material was processed by Tangential Flow Filtration (TTF) with a 1 K membrane until the total volume was reduced to 54 L.Water (120 L) was added in two portions and the solution was concentrated to 52 L by continued TTF. The aqueous solution (30 L of 52 L) was purified by chromatography using the following protocol: The aqueous solution was brought to three times of its volume (30 L→90l_) with 20% IPA in aqueous ammonium acetate solution (50 mM). The diluted solution was applied to a 38 L HP20SS resin column at 1.5 L/min. The column was eluted with IPA solution in aqueous 50 mM ammonium acetate (25%→30%→35%, 60 L each concentration).
Fractions (approximately 11 L) were collected and analyzed by HPLC. The fractions with HPLC purity less than 80% were combined and purified again using the same method. The key fractions from both chromatographic separations (with HPLC purity >80%) were combined and acidified with concentrated HCI to pH 2-3. The resulting solution was desalted on an ion exchange column (HP20SS resin, 16 L) which was eluted with WFI (until conductivity = 4.8 μS) followed by IPA in WFI (36 L 10%→ 40 L 60%). The yellow band which was eluted with 60% IPA (approximately 19L) was collected, adjusted to pH 2-3 with concentrated HCI and lyophilized to yield 636.5 g of Compound 49 (HPLC purity of 87.0%). MS m/z 1680.7 (M + H)+.
……………………………..
see formulation
| WO2012162567A1 | May 24, 2012 | Nov 29, 2012 | Cubist Pharmaceuticals, Inc. | Cb-183,315 compositions and related methods |
References
- http://www.cubist.com/downloads/Surotomycin-Fact-Sheet-13013.pdf
-
- Cubist Pharmaceuticals. Cubist products and pipeline. Available online: http://www.cubist.com/products/(accessed on 15 April 2013).
- Cubist Pharmaceuticals. Study of CB-183,315 in patients with Clostridium difficile associated diarrhea.Available online: http://www.clinicaltrials.gov/ct2/show/NCT01597505 (accessed on 15 April 2013).
- Cubist Pharmaceuticals. A study of CB-183,315 in patients with Clostridium difficile associated diarrhea.Available online: http://www.clinicaltrials.gov/ct2/show/NCT01598311 (accessed on 15 April 2013).
- Mascio, C.T.M.; Mortin, L.I.; Howland, K.T.; van, P.A.D.G.; Zhang, S.; Arya, A.; Chuong, C.L.; Kang, C.; Li, T.; Silverman, J.A. In vitro and in vivo characterization of CB-183,315, a novel lipopeptide antibiotic for treatment of Clostridium difficile. Antimicrob. Agents Chemother. 2012, 56, 5023–5030, doi:10.1128/AAC.00057-12.
-
WO2012162567A1 May 24, 2012 Nov 29, 2012 Cubist Pharmaceuticals, Inc. Cb-183,315 compositions and related methods
-
WO2001097851A2 * Jun 18, 2001 Dec 27, 2001 Cubist Pharm Inc Compositions and methods to improve the oral absorption of antimicrobial agents WO2010075215A1 Dec 18, 2009 Jul 1, 2010 Cubist Pharmaceuticals, Inc. Novel antibacterial agents for the treatment of gram positive infections WO2011063419A2 * Nov 23, 2010 May 26, 2011 Cubist Pharmaceuticals Inc. Lipopeptide compositions and related methods





















Sorry, the comment form is closed at this time.