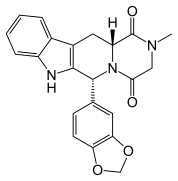
Tadalafil
GF-196960, IC-351, Cialis
6R–trans)-6-(1,3-benzodioxol-5-yl)- 2,3,6,7,12,12a-hexahydro-2-methyl-pyrazino [1′, 2′:1,6] pyrido[3,4-b]indole-1,4-dione
Pyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione,6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6R-trans)-; (6R,12aR)-6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-ethylpyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione; GF 196960; Adcirca;
171596-29-5 casno
Molecular Weight:
389.40
Molecular Formula:C22H19N3O4
GlaxoSmithKline (Originator), Lilly Icos (Marketer), Lilly (Licensee), Lilly Icos (Licensee)
Launched-2003
Tadalafil is currently marketed as Cialis. Cialis was developed by Eli Lilly as a treatment for impotence. In this capacity, it is reported that tadalafil functions by inhibiting the formation of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). The inhibition of PDE5 presumably lessens impotence by increasing the amount ot c(iMP, resulting in smooth muscle relaxation and increased blood flow.

Tadalafil is a PDE5 inhibitor marketed in pill form for treating erectile dysfunction (ED) under the name Cialis, and under the name Adcirca for the treatment of pulmonary arterial hypertension. In October 2011 the U.S. Food and Drug Administration (FDA) approved Cialis for treating the signs and symptoms of benign prostatic hyperplasia (BPH) as well as a combination of BPH and erectile dysfunction (ED) when the conditions coincide. It initially was developed by the biotechnology company ICOS, and then again developed and marketed world-wide by Lilly ICOS, LLC, the joint venture of ICOS Corporation and Eli Lilly and Company. Cialis tablets, in 2.5 mg, 5 mg, 10 mg, and 20 mg doses, are yellow, film-coated, and almond-shaped. The approved dose for pulmonary arterial hypertension is 40 mg (two 20-mg tablets) once daily.
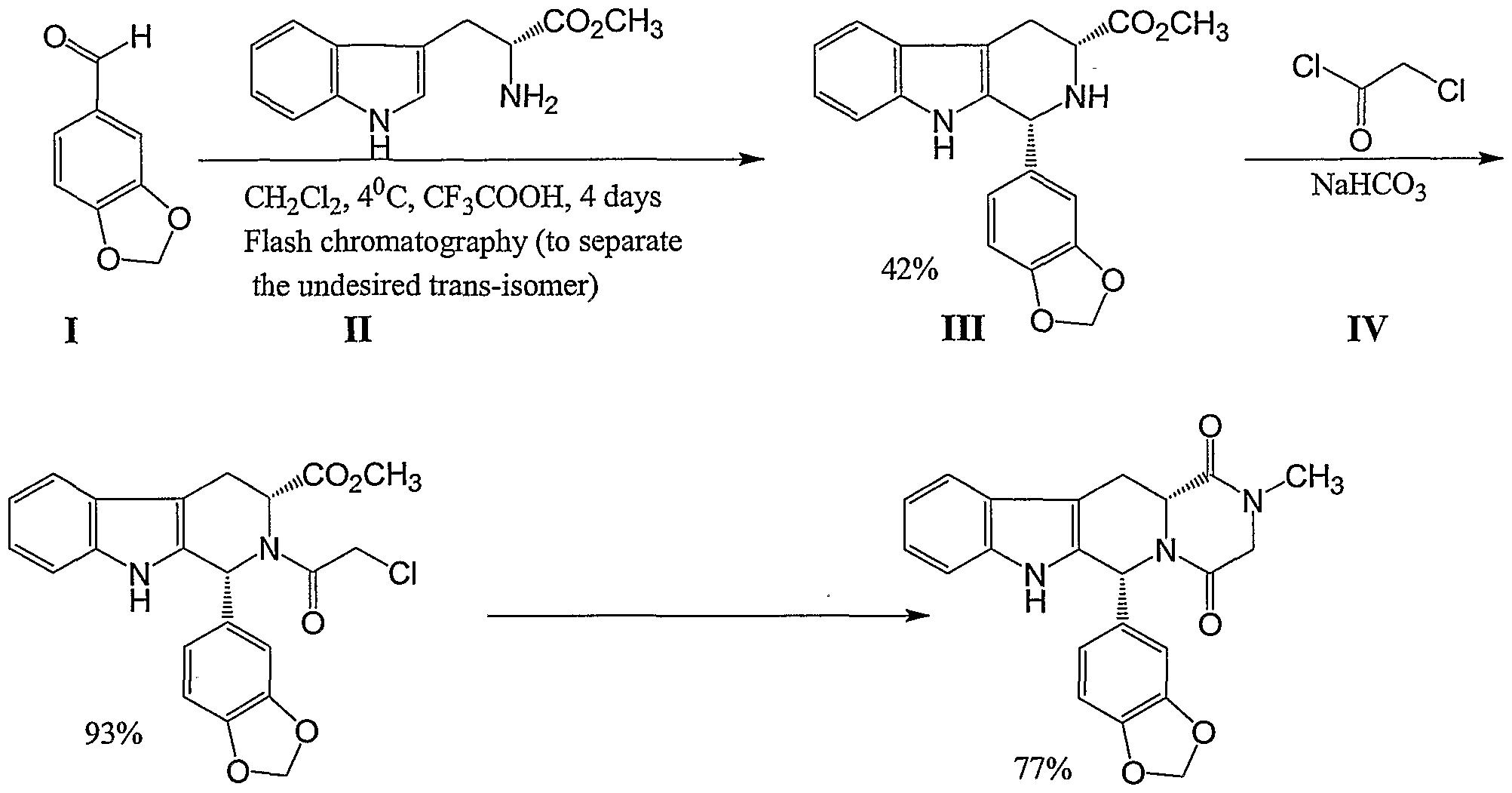
Tadalafil can be prepared via a series of intermediates. One synthesis scheme is illustrated in Scheme 1: Scheme 1
U.S. Patent No. 5,859,006 describes the synthesis of the tadalafil intermediate (Compound III) from D-tryptophan methyl ester (Compound II) and piperonal (Compound I) using trifluoroacetic acid and dichloromethane, a halogenated solvent. Compound III is then reacted with chloroacetyl chloride (Compound IV) and chloroform, providing another intermediate of tadalafil (Compound V). WO 04/011463 describes a process of preparing tadalafil intermediates from D-tryptophan methyl ester HCl salt and piperonal by refluxing the reagents in isopropyl alcohol; the obtained intermediate is reacted with chloroacetyl chloride and THF, resulting in another intermediate of tadalafil.
Tadalafil is also manufactured and sold under the name of Tadacip by the Indian pharmaceutical company Cipla in doses of 10 mg and 20 mg.
On November 21, 2003 the FDA approved tadalafil (as Cialis) for sale in the United States as the third ED prescription drug pill (after sildenafil citrate(Viagra) and vardenafil (Levitra)). Like sildenafil and vardenafil, tadalafil is recommended as an ‘as needed’ medication. Cialis is the only one of the three that is also offered as a once-daily medication.
Moreover, tadalafil was approved in May 2009 in the United States for the treatment of pulmonary arterial hypertension and is under regulatory review in other regions for this condition. In late November 2008, Eli Lilly sold the exclusive rights to commercialize tadalafil for pulmonary arterial hypertension in the United States to United Therapeutics for an upfront payment of $150 million.

The FDA’s approval of Viagra (Sildenafil) on March 27, 1998 was a ground-breaking commercial event for the treatment of ED, with sales exceedingUS$1 billion. Subsequently, the FDA approved Levitra (vardenafil) on August 19, 2003, and Cialis (tadalafil) on November 21, 2003.
Cialis was discovered by Glaxo Wellcome (now GlaxoSmithKline) under a partnership between Glaxo and ICOS to develop new drugs that began in August 1991. [1][2] In 1993, the Bothell, Washington biotechnology company ICOS Corporation began studying compound IC351, a phosphodiesterase type 5 (PDE5) enzyme inhibitor. In 1994, Pfizer scientists discovered that sildenafil, which also inhibits the PDE5 enzyme, caused penile erection in men participating in a clinical study of a heart medicine. Although ICOS scientists were not testing compound IC351 for treating ED, they recognized its potential usefulness for treating that disorder. Soon, in 1994, ICOS received a patent for compound IC351 (structurally unlike sildenafil and vardenafil), and Phase 1 clinical trials began in 1995. In 1997, the Phase 2 clinical studies were initiated for men experiencing ED, then progressed to the Phase 3 trials that supported the drug’s FDA approval. Although Glaxo had an agreement with ICOS to share profits 50/50 for drugs resulting from the partnership, Glaxo let the agreement lapse in 1996 as the drugs developed were not in the company’s core markets.[3]

In 1998, ICOS Corporation and Eli Lilly and Company formed the Lilly ICOS, LLC, joint venture company to further develop and commercialize tadalafil as a treatment for ED. Two years later, Lilly ICOS, LLC, filed a new drug application with the FDA for compound IC351 (under the tadalafil generic name, and the Cialis brand name). In May 2002, Lilly ICOS reported to the American Urological Association that clinical trial testing demonstrated that tadalafil was effective for up to 36 hours, and one year later, the FDA approved tadalafil. One advantage Cialis has over Viagra and Levitra is its 17.5-hour half-life (thus Cialis is advertised to work for up to 36 hours, after which time there remains approximately 25 percent of the absorbed dose in the body) when compared to the four-hour half–life of sildenafil (Viagra).
In 2007, Eli Lilly and Company bought the ICOS Corporation for $2.3 billion. As a result, Eli Lilly owned Cialis and then closed the ICOS operations, ending the joint venture and firing most of ICOS’s approximately 500 employees, except for 127 employees of the ICOS biologics facility, which subsequently was bought by CMC Biopharmaceuticals A/S (CMC).
Persons surnamed “Cialis” objected to Eli Lilly and Company’s so naming the drug, but the company has maintained that the drug’s trade name is unrelated to the surname.[4]
On October 6, 2011, the U.S. FDA approved tadalafil [5] to treat the signs and symptoms of benign prostatic hyperplasia (BPH). BPH is a condition in males in which the prostate gland becomes enlarged, obstructing the free flow of urine. Symptoms may include sudden urges to urinate (urgency), difficulty in starting urination (hesitancy), a weak urine stream, and more frequent urination- especially at night. The FDA has also approved tadalafil for treatment of both BPH and erectile dysfunction (ED) where the two conditions co-exist.
Although available since 2003 in 5, 10, 20 mg dosage, in late 2008/early 2009, the U.S. FDA approved the commercial sale of Cialis in 2.5 mg dosage as a one-a-day treatment for ED. The 2.5 mg dose avoids earlier dispensing restrictions on higher dosages. The price of the 5 mg and 2.5 mg are often similar, so some people score and split the pill.[6] The manufacturer does not recommend splitting.
Moreover, tadalafil (Adcirca) 40 mg was approved in 2009 in the United States and Europe (and 2010 in Canada and Japan) as a once-daily therapy to improve exercise ability in patients withpulmonary arterial hypertension. In patients with pulmonary arterial hypertension, the pulmonary vascular lumen is decreased as a result of vasoconstriction and vascular remodeling, resulting in increased pulmonary artery pressure and pulmonary vascular resistance. Tadalafil is believed to increase pulmonary artery vasodilation, and inhibit vascular remodeling, thus lowering pulmonary arterial pressure and pulmonary vascular resistance. Right heart failure is the principal consequence of pulmonary arterial hypertension.
On October 6, 2011, the U.S. FDA approved tadalafil [6] to treat the signs and symptoms of benign prostatic hyperplasia (BPH). BPH is a condition in males in which the prostate gland becomes enlarged, obstructing the free flow of urine. Symptoms may include sudden urges to urinate (urgency), difficulty in starting urination (hesitancy), a weak urine stream, and more frequent urination- especially at night. The FDA has also approved tadalafil for treatment of both BPH and erectile dysfunction (ED) where the two conditions co-exist.
Tadalafil has been used in approximately 15,000 men participating in clinical trials, and over eight million men worldwide (primarily in the post-approval/post-marketing setting). The most commonside effects when using tadalafil are headache, stomach discomfort or pain, indigestion, burping, acid reflux. back pain, muscle aches, flushing, and stuffy or runny nose. These side effects reflect the ability of PDE5 inhibition to cause vasodilation (cause blood vessels to widen), and usually go away after a few hours. Back pain and muscle aches can occur 12 to 24 hours after taking the drug, and the symptom usually disappears after 48 hours.
In May 2005, the U.S. Food and Drug Administration found that tadalafil (along with other PDE5 inhibitors) was associated with vision impairment related to NAION (nonarteritic anterior ischemic optic neuropathy) in certain patients taking these drugs in the post-marketing (outside of clinical trials) setting. Most, but not all, of these patients had underlying anatomic or vascular risk factors for development of NAION unrelated to PDE5 use, including: low cup to disc ratio (“crowded disc”), age over 50, diabetes, hypertension, coronary artery disease, hyperlipidemia and smoking. Given the small number of NAION events with PDE5 use (fewer than one in one million), the large number of users of PDE5 inhibitors (millions) and the fact that this event occurs in a similar population to those who do not take these medicines, the FDA concluded that they were not able to draw a cause and effect relationship, given these patients underlying vascular risk factors or anatomical defects. However, the label of all three PDE5 inhibitors was changed to alert clinicians to a possible association.
In October 2007, the FDA announced that the labeling for all PDE5 inhibitors, including tadalafil, requires a more prominent warning of the potential risk of sudden hearing loss as the result of postmarketing reports of deafness associated with use of PDE5 inhibitors.[7]
Selectivity compared with other PDE5 inhibitors
Tadalafil, sildenafil, and vardenafil all act by inhibiting the PDE5 enzyme. These drugs also inhibit other PDE enzymes. Sildenafil and vardenafil inhibit PDE6, an enzyme found in the eye, more than tadalafil.[9] Some sildenafil users see a bluish tinge and have a heightened sensitivity to light because of PDE6 inhibition.[3] Sildenafil and vardenafil also inhibit PDE1 more than tadalafil.[9]PDE1 is found in the brain, heart, and vascular smooth muscle.[9] It is thought that the inhibition of PDE1 by sildenafil and vardenafil leads to vasodilation, flushing, and tachycardia.[9] Tadalafil inhibits PDE11 more than sildenafil or vardenafil.[9] PDE11 is expressed in skeletal muscle, the prostate, the liver, the kidney, the pituitary gland, and the testes.[9] The effects on the body of inhibiting PDE11 are not known.[9]
In the United States, the FDA relaxed rules on prescription drug marketing in 1997, allowing advertisements targeted directly to consumers.[10] Lilly-ICOS hired the Grey Worldwide Agency in New York, part of the Grey Global Group, to run the Cialis advertising campaign.[11] Marketers for Cialis has taken advantage of its greater duration compared to its competitors in advertisements for the drug; Stuart Elliot of The New York Times opined: “The continuous presence of women in Cialis ads is a subtle signal that the drug makes it easier for them to set the pace with their men, in contrast to the primarily male-driven imagery for Levitra and Viagra.”[11] Iconic themes in Cialis ads include couples in bathtubs and the slogan “When the moment is right, will you be ready?”[11] Cialis ads were unique among the ED drugs in mentioning specifics of the drug.[12] As a result, Cialis ads were also the first to describe the side effects in an advertisement, as the FDA requires advertisements with specifics to mention side effects. One of the first Cialis ads aired at the 2004 Super Bowl.[12] Just weeks before the Super Bowl, the FDA required more possible side effects to be listed in the advertisement, including priapism.[12] Although many parents objected to the Cialis ad being aired during the Super Bowl, Janet Jackson‘s halftime “wardrobe malfunction” overshadowed Cialis.[12] In January 2006, the Cialis ads were tweaked, adding a doctor on screen to describe side effects and only running ads where more than 90 percent of the audience are adults, effectively ending Super Bowl ads.[10] In 2004, Lilly-ICOS, Pfizer, and GlaxoSmithKline spent a combined $373.1 million to advertise Cialis, Viagra, and Levitra respectively.[12] Cialis has sponsored many golf events, including the America’s Cup and the PGA Tour, once being title sponsor of the PGA Tour Western Open tournament.[13]
CIALIS (tadalafil) is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). Tadalafil has the empirical formula C22H19N3O4 representing a molecular weight of 389.41. The structural formula is:
 |
The chemical designation is pyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)2,3,6,7,12,12a-hexahydro-2-methyl-, (6R,12aR)-. It is a crystalline solid that is practically insoluble in water and very slightly soluble in ethanol.
CIALIS is available as almond-shaped tablets for oral administration. Each tablet contains 2.5, 5, 10, or 20 mg of tadalafil and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, hypromellose, iron oxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate, talc, titanium dioxide, and triacetin.
Tadalafil, (6R-trans)-6-(l,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2- methyl-pyrazino[r,2′:l,6]pyrido[3,4-b]indole-l,4-dione, with the structural formula shown below, is a white crystalline powder. (CAS# 171596-29-5). Tadalafil is a potent and selective inhibitor of the cyclic guanosine monophosphate (cGMP) – specific phosphodiesterase enzyme, PDE5. The inhibition of PDE5 increases the amount of cGMP, resulting in smooth muscle relaxation and increased blood flow. Tadalafil is therefore currently used in the treatment of male erectile dysfunction, and is commercially available as CIALIS ®.
Tadalafil U.S. Patent No. 5,859,006 describes the synthesis of tadalafil via the cyclization of TDCL (i.e., cis-methyl l,2,3,4-tetrahydro-2-chloroacetyl-l-(3,4- methylenedioxyphenyl)-9H-pyrido[3,4-b]mdole-3-carboxylate) using methylamine by purification by flash chromatography, followed by subsequent crystallization from methanol. Crude tadalafil typically requires additional purification steps, such as multiple extractions, crystallization, and/or flash chromatography, to remove the impurities present in the compound after synthesis is complete. Such purification processes increase the cost of producing tadalafil. Also, when repeating the US ‘006 process, about 250 volumes of methanol were necessary for the crystallization step
Tadalafil can be prepared via a series of intermediates. One synthesis for preparing tadalafil is illustrated below in Scheme I:
SCHEME I
U.S. Patent No. 5,859,006 discloses the synthesis of a tadalafil intermediate
(Compound III) from D-tryptophan methyl ester (Compound II) and piperonal (Compound
I) using trifluoroacetic acid and dichloromethane, a halogenated solvent. Compound III is then reacted with chloroacetyl chloride (Compound IV) and chloroform to provide another intermediate of tadalafil (Compound V).
WO 2004/011463 discloses a process of preparing tadalafil intermediates from D-tryptophan methyl ester HCl salt and piperonal by refluxing the reagents in isopropyl alcohol, reacting the intermediate thus obtained with chloroacetyl chloride and tetrahydrofuran (THF) to provide another intermediate of tadalafil.
WO 2006/110893 discloses a process for the preparation of methyl ester intermediate (Compound III), and tadalafil using the methyl ester intermediate (CompoundII).
U.S. Patent Application Publication No. 2006/0258865 Al discloses a synthesis of the tadalafil intermediate (Compound III) from D-tryptophan methyl ester
(Compound II) and piperonal (Compound I) using a dehydrating agent selected from Na2SO4, K2SO4, MgSO4, CaSO4, CaCl2, molecular sieve or mixtures thereof and a high boiling solvent such as N,N-Dimethyl acetamide. Compound III is then reacted with chloroacetyl chloride (Compound IV) in the presence of a base such as NaHCO3 and an organic solvent such as dichloromethane, providing another intermediate of tadalafil (Compound V), which is further reacted with aqueous methyl amine solution to provide tadalafil.
………………………………………….
Scheme II and III.
……………………………………………………………………………………………………a compound of .Formula I
SCHEME III
SCHEME IV
EXAMPLE l
The reaction scheme of this example is generally shown below in SchemeIV.
SCHEME IV
Compound – 1 Compound – II
Into a clean dry glass flask charged with ethanol (250 ml) under a nitrogen atmosphere was added Compound 1 (25 g) under stirring. The reaction mass was cooled to 0 to 50C and monomethylamine gas was purged into the reaction mixture for about 2 hours while maintaining the temperature between 0 to 50C. The temperature was raised to 75 to 😯0C and the reaction mixture was stirred under reflux for 2 hours. The reaction mixture was then cooled to 0 to 5°C and monomethylamine gas was again purged into the reaction mixture at 0 to 5°C. The temperature was again raised to 75 to 800C and stirred for about 1 hour. The reaction mixture was concentrated under vacuum to about 1/3 its original volume, cooled to 5 to 1O0C and stirred for 1 hour at this temperature. The solids were filtered and washed with chilled ethanol (50 ml). The wet solids were dried under vacuum for 6 hours.
Yield: 25g; Mp: 202-206.70C
Specific rotation (25°C) :+44.0 ( C=l% in DMSO)
13C NMR, DMSO-D6 : 25.78, 25.92, 57.89, 57.98, 101.17, 108.09, 108.32,
109.08, 111.48, 117.82, 118.62, 122.23, 122.97, 126.97, 135.97, 136.22, 136.55, 146.99,
147.48, 173.13
1H NMR, DMSO-D6, 300 MHz, Delta values: 2.6(m,lH), 2.7(m,3H),
2.8(d,lH), 3.0(d,lH), 3.6(bs,lH), 5.1(m,lH), 6.0(s,3H), 6.9-7.1(m, 5H), 7.2(d,lH),
7.4(d,lH), 7.8(bs, IH), 10.3(s, IH)
EXAMPLE 2
The reaction scheme of this example is generally shown below in SchemeV.
SCHEME V
Formula III Formula II
Into a clean dry flask charged with dichloromethane (200 ml) was added
Compound II (25 g) obtained in Example 1 under stirring at 25 to 300C. Next, triethylamine (16.11 g) was added to the reaction mixture and stirred for 30 minutes at 20 to 300C. The reaction mixture was cooled to 0 to 5°C and a solution of chloroacetyl chloride (12.93 g) in chloroform (50 ml) was added to the reaction mixture while maintaining temperature between -5 to 50C. The reaction mixture was stirred at -5 to 5°C for about 2 hours. Saturated aqueous sodium bicarbonate solution (50 ml) was added to the reaction mass slowly and the temperature of the reaction mixture was raised to 25 to 300C. The lower organic layer was separated and washed twice with water (75 ml). The chloroform extract was dried over anhydrous sodium sulfate. The organic layer was concentrated under vacuum until a thick yellow slurry was obtained. The slurry was cooled to 0 to5°C. The solids obtained were filtered and washed with 50 ml chilled chloroform. The wet product was dried at 750C under vacuum for 6 hours.
Yield: 22.5 g; HPLC Purity: 97%; Mp: 180-1820C
Specific rotation(25°C): -154.3(C=1% in DMSO)
13C. NMR(DMSO-Do, 300 MHZ)= 21.11, 25.88, 44.207, 51.60, 53.95,
101.16,107.66 109.56, 111.38, 118.36, 118.75, 121.58,122.74, 126.30, 130.31, 134.13,
136.57, 146.66, 147.03,167.43, 168.45
1H. NMR (CDC13, 300 MHZ):2.4(bs,3H), 3.1(m,lH), 3.8(m,lH),
4.3(bs,2H), 4.9(m,lH), 5.4(m,lH), 5.9(s,2H), 6.6-6.8(m,3H), 6.9(bs,lH), 7.1-7.3(m,3H),
7.6(d, IH), 7.7(bs,lH)
1H. NMR (DMSO-D6, 300 MHZ): 2.0 (bs,3H), 2.9(m,lH), 3.4(m,lH),
4.5(m,lH), 4.8(m,lH), 4.9(m,lH), 6.0(m,2H), 6.4-6.8(m,4H), 6.9-7.2(m,2H), 7.3(d, IH),
7.4(bs,lH), 7.5(d,lH), 10.8(s,lH)
EXAMPLE 3
The reaction scheme of this example is generally shown below in SchemeVI.
SCHEME VI
Formula II Formula I
Into a clean dry round bottom (RB) flask was charged tetrahydrofuran
(THF) (175 ml) under a nitrogen blanket and then cooled to -35 to -400C. Next 92 ml n- butyllithium (1.6 m solution in hexane) was added while maintaining the temperature between -35 to -400C. After the addition was complete, the reaction mixture was stirred at -35 to -400C for 15 minutes. A solution of compound of formula II (22.5 g) obtained in Example 2 in THF (75 ml) was prepared and slowly added to the reaction mixture while maintaining the temperature between -35 to -400C. After the addition was complete, the reaction mixture was stirred at -35 to -400C for 2.5 hours. Saturated aqueous ammonium chloride solution (25 ml) and 50 ml ethyl acetate was added to the reaction mixture at -35 to -400C. The temperature was raised to 25 to 300C and the two layers formed were separated. The upper organic layer was collected. The lower aqueous layer was thrice extracted with ethyl acetate (25 ml). The organic layers were combined together and washed with water (50 ml). The organic extract was dried over anhydrous sodium sulfate and concentrated under vacuum to obtain crude tadalafil as a dark brown solid. [0058] Yield: 22 g; HPLC Purity: 50%.
EXAMPLE 4
Purification of crude tadalafil
The crude tadalafil (22 g) obtained in Example 3 was suspended in 110 ml methanol and stirred for 1 hour at 25 to 300C. The solids obtained were filtered and washed with 25 ml chilled methanol. The wet product was dried at 600C under vacuum for 6 hours. This was further purified by using isopropyl alcohol. Yield: 9 g; HPLC Purity: >99.5%.
EXAMPLE 5
The reaction scheme of this example is generally shown below in SchemeVII.
Scheme VII
Formula VI where R = -OCH3 Formula VIA [0062] Into a clean dry RB flask charged with methanol (1900 ml) was added D- tryptophan methyl ester (190 g) under stirring at 25 to 300C. The reaction mixture was cooled to 0 to 50C. Monomethylamine gas was purged into the reaction mixture at 0 to 5°C for about 5-7 hours under stirring. The temperature of the reaction mixture was slowly raised to about 25 to 3O0C and stirred at this temperature for 5-7 hours. The reaction mixture was concentrated under vacuum to distill out the solvent. Diisopropyl ether (950 ml) was added and cooled to 25 to 3O0C under stirring for 1-2 hrs. The solids obtained were filtered, washed with Diisopropyl ether and dried under vacuum. [0063] MP: 122.4-1240C; Yield: 150 g (78.9 % w/w).
Specific rotation(25°C): +12.5 (C=I % in DMSO)
13 C NMR (300 MHZ,DMSO-D6): 25.71, 31.40, 55.67, 110.93, 111.55,
118.42, 118.73, 121.09, 123.95, 127.66,136.44, 175.39.
1H NMR (300 MHZ,DMSO-D6): 1.6(bs,2H), 2.5(m,3H), 2.8(m,lH),
3.1(m,lH), 3.4(m, IH), 6.9-7.2(m,3H), 7.3(d,lH), 7.5(d,lH), 7.8(bs,lH), 10.8(bs,lH)
EXAMPLE 6
The reaction scheme of this example is generally shown below in Scheme VIII.
SCHEME VIII
Formula VIA Formula VII
Into a clean, dry flask charged with methylene dichloride (MDC) (1000 ml) was added D-tryptophan methyl amide, the compound of Formula VIA (50 g), and piperonal, the compound of Formula VII (31.09 g), under stirring at 25 to 300C. The reaction mixture was cooled to 0 to 5°Cunder nitrogen atmosphere. Trifluoroacetic acid (85.3 g) was dissolved in MDC (250 ml) and the solution was slowly added to the reaction mixture at 0 to 5°C. The temperature of the reaction mixture was raised to 20 to 300C and stirred at this temperature for 14-16 hours. The reaction was monitored by TLC, workup was done as follows, the pH of the reaction mixture was adjusted to 8-9 using sodium carbonate solution under stirring, the two layers were settled, separated and the lower MDC layer was washed with water. The MDC layer was then dried over anhydrous sodium sulfate. The reaction mass was concentrated under vacuum at 40 to 5O0C to remove the solvent. The compound was precipitated using ethyl acetate, the solids were filtered, washed with ethyl acetate and dried.
Yield: 52.5 g; Yield: 105% w/w, HPLC Purity: 71% cis and 27% trans isomer (HPLC).
EXAMPLE 7
The reaction scheme of this example is generally shown below in Scheme IX.
SCHEME IX
1]CICOCH2C1 2]crystn
Formula H
Into a clean dry flask charge with dichloromethane (400 ml) under a nitrogen atmosphere was added the compound of Formula III obtained in Example 6 and triethylamine (28.96 g) under stirring at 20 to 3O0C. The reaction mixture was then cooled to 0 to 50C. A mixture of chloroacetyl chloride (25.85 g) in dichloromethane (100 ml) was prepared and slowly added to the reaction mixture while maintaining the temperature between -5 to 50C in 1-2 hrs. The reaction mixture was stirred at 0 to 50C for 30 min and then saturated sodium bicarbonate solution (100 ml) was added at 5 to 100C under stirring. The temperature of the reaction mixture was raised to 25 to 300C and stirred at this temperature for 15 minutes. The layers were then separated. The lower MDC layer was collected, washed twice with 100 ml water and dried over anhydrous sodium sulfate. The
MDC layer was concentrated to distill out MDC until a stirrable mass was left behind. The mass was cooled to 25-3O0C and filtered, washed, to yield off-white to light yellow colored solids. The resulted product was the cis isomer, the trans isomer left behind in the mother liquor.
Yield = 25.5 g (50%w/w); HPLC Purity: > 97%.
The physical and spectral data was similar to that obtained in Example 2.
EXAMPLE 8
The reaction scheme of this example is generally shown below in SchemeX.
SCHEME X
Formula II
Into a clean dry round bottom (RB) flask was charged THF (1625 ml) under a nitrogen blanket and then cooled to -35 to -400C. Next, 505 ml n-butyllithium (1.6 m solution in hexane) was added while maintaining the temperature between -35 to -4O0C. After the addition was complete, the reaction mixture was stirred at -35 to -4O0C for 15 minutes. 72 ml diisopropyl amine was then added at -35 to -400C and then stirred at 0-50C for 1 hr. A solution of Compound of formula II (125 g) obtained in Example 7 in THF (625 ml) was prepared and slowly added to the reaction mixture while maintaining the temperature between -40 to -5O0C. After the addition was complete, the reaction mixture was stirred at -35 to -400C for 2-6 hours. Saturated aqueous ammonium chloride solution (250 ml) and ethyl acetate (125 ml) was added to the reaction mixture at -35 to -400C. The temperature was raised to 25 to 300C and the two layers formed were separated. The upper organic layer was collected. The lower aqueous layer was extracted with ethyl acetate (65 ml). The organic layers were combined together and distilled. Isopropyl alcohol (1250 ml) was added and the distillation was continued. A mixture of methanol (250 ml) and isopropanol (375 ml) were added and crude tadalafϊl was obtained upon cooling. The crude product was filtered, washed with water and dried. [0076] Yield: 60 g; (48% w/w); HPLC Purity: >99%.
EXAMPLE 9
Purification of crude Tadalafil
The crude tadalafil obtained in Example 8 was suspended in methanol (600 ml) and stirred for 1 hour at reflux. The mixture was cooled and the solids obtained were filtered and washed with chilled methanol (60 ml). The wet product was dried at under vacuum.
Yield: 56 g; HPLC Purity: 99.8%.
……………………………………………………………………………………
Beilstein J. Org. Chem. 2011, 7, 442–495.
http://www.beilstein-journals.org/bjoc/single/articleFullText.htm?publicId=1860-5397-7-57#S28
A different approach was used in the synthesis of the phosphodiesterase inhibitor tadalafil (132, Cialis) starting from commercially available (D)-tryptophan methyl ester to form the indolopiperidine motif 135 via a Pictet–Spengler reaction followed by a double condensation to install the additional diketopiperazine ring (Scheme 28) [38,39].
To achieve the high levels of cis selectivity required from the Pictet–Spengler reaction, an extensive investigation of solvents and the influence of additives was undertaken [40]. It was identified that the use of a specific 23 mol % of benzoic acid significantly increased the cis/trans ratio from a base level of 55:45 to 92:8 (16 h reaction time at ambient temperature) in an overall yield of 86%. It was also determined that more polar solvents such as acetonitrile and nitromethane preferentially solvated the trans product and thereby allowed the isolation of the ciscompound by precipitation. It was also shown that by heating the reaction mixture under reflux the product distribution could be driven to the thermodynamically more favoured cis isomer having both the ester and the piperonyl moiety in equatorial positions. Hence, after heating under reflux for 8 h the cis/trans ratio was found to be 99:1 and the product could be isolated in an overall yield of 91%. This work represents an impressive example of a well considered and executed process optimisation study.
………………………………
The process disclosed in the patent US 5 859 006 (Scheme 1) involves condensation of D-tryptophan methyl ester with a piperonal derivative to yield a compound of formula (II). After conversion into a thioamide derivative of formula (III), cyclization occurs in presence of both an alkylating and reducing agents to provide a tetrahydro-β-carboline derivative of formula (IV), which on treatment with chloroacetyl chloride and methyl amine, gives Tadalafil. The compound of formula (IV) can also be obtained in one step, after separation of the other diastereoisomer, by a Pictet Spengler reaction between D-tryptophan methyl ester and piperonal in presence of an acid, such as trifluoroacetic acid.

- The patent application WO2007/10038 discloses the reaction of D-tryptophan with piperonal to provide a tetrahydro-β-carboline acid that was cyclised to Tadalafil in presence of a sarcosine derivative.
The patent application WO2007/1107 discloses the reaction of D-tryptophan methyl amide with piperonal, to provide an intermediate that after reaction with chloroacetyl chloride cyclises to Tadalafil in presence of butyllithium.
Thus, the active substance prepared by the processes known up till now can only be obtained in a satisfactory quality after running through a large number of process steps. Moreover a toxic alkylating agent, such as methylamine, is often used.
Example 1
- (1R,3R)-methyl-1,2,3,4-tetrahydro-2-(2-(benzyl(methyl)amino)acetyl)-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3-carboxylate (VII)
-
A 50 mL three-necked flask fitted with thermometer and reflux condenser was charged with (1R,3R)-methyl 1,2,3,4-tetrahydro-2-chloroacetyl-1-(3,4-methylenedioxyphenyl)-9H-pyrido [3,4-b] indole-3-carboxylate (VI) (1.39 g, 3.26 mmol), DMA (5.33 mL), K2CO3 (0.5 g, 3.6 mmol) and N-benzylmethylamine (0.41 mL, 3.26 mmol). The resultant solution was stirred at room temperature. After 2 hours, the mixture was poured in brine (20 mL) and extracted with isopropyl acetate. The combined organic phases were washed with brine (3 x 5 mL), dried over sodium sulfate and concentrated to a residue under reduced pressure, affording 1.5 g of the desired product (VII), as a white solid. Yield: 70%.
1H NMR (d6-DMSO 300 MHz, 298K) 2.24 (s, 3H), 2.94-3.00 (m, 5H), 3.44-3.68 (m, 3H), 5.56 (bd, J = 6.4, 1H), 5.95 (s, 1H), 5.96 (s, 1H), 6.55 (bd, J = 7.4, 1H), 6.75 (bs, 1H), 6.77 (d, J = 8.0, 1H), 6.84 (bs, 1H), 7.05 (td, J = 7.4, 0.9, 1H), 7.12 (td, J = 7.5, 1.2, 1H), 7.17-7.32 (m, 6H), 7.56 (d, J = 7.7, 1H), 10.76 (bs, 1H)
13C NMR (d6-DMSO 75.4 MHz, 298K) 21.9, 42.5, 51.3, 51.9, 52.4, 61.0, 61.7, 101.5, 107.0, 108.0, 109.8, 111.8, 118.5, 119.2, 122.0, 123.0, 126.7, 127.7, 128.7, 129.6, 131.1, 134.7, 137.1, 138.6, 147.1, 147.5, 170.6, 171.5
Example 2
- (6R-trans)-6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-pyrazino [1′,2′:1,6] pyrido [3,4-b] indole-1,4-dione (Tadalafil) (I)
Under H2 atmosphere (3 atm) and magnetic stirring, Raney® Ni (2800 slurry in water, 0.0276 g, 0.47 mmol), previously washed with methanol (3 times), was added to a solution of (1R,3R)-methyl-1,2,3,4-tetrahydro-2-(2-(benzyl(methyl)amino)acetyl)-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3-carboxylate (VII) (3.00 g, 4.70 mmol) in DMA (21.3 mL). The mixture was heated at 90°C for 17 hours and then cooled to room temperature. The suspension was filtered over a pad of Celite® and the resulting solutionand the resulting solution was concentrated until 6 mL. Methanol (12 mL) was added and the solid which was so obtained was filtered over Buchner, washed with methanol (4 mL) and oven-dried under reduced pressure for 2 hours, affording 1.3 g of the title compound, as a white solid. Yield: 70%
1H NMR (d6-DMSO 300 MHz, 298K): 2.91-3.00 (m, 4H), 3.32 (s, 1H), 3.47-3.54 (dd, J = 4.6, 11.3, 1H), 3.93 (d, J = 17.1, 1H), 4.17 (d, J = 17.1, 1H), 4.35-4.40 (dd, J = 4.27, 11.6, 1H), 5.91 (s, 2H), 6.11 (s, 1H), 6.76 (s, 2H), 6.85 (s, 1H), 6.98-7.06 (m, 2H), 7.28 (d, J = 7.9, 1H), 7.52 (d, J = 7.3, 1H), 11.0 (s, 1H)
13C NMR (d6-DMSO 75.4 MHz, 298K) 23.8, 33.4, 52.0, 55.9, 56.1, 101.5, 105.3, 107.6, 108.6, 111.9, 118.7, 119.5, 119.9, 121.8, 126.4, 134.5, 136.8, 137.6, 146.7, 147.6, 167.1, 167.5
References
- Daugan, A; Grondin P, Ruault C, Le Monnier de Gouville AC, Coste H, Kirilovsky J, Hyafil F, Labaudinière R (October 9, 2003). “The discovery of tadalafil: a novel and highly selective PDE5 inhibitor. 1: 5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione analogues”. Journal of Medicinal Chemistry 46 (21): 4525–32. doi:10.1021/jm030056e . PMID 14521414.
- Richards, Rhonda (September 17, 1991). “ICOS At A Crest On Roller Coaster”. USA Today. p. 3B.
- Ervin, Keith (June 21, 1998). “Deep Pockets + Intense Research + Total Control = The Formula — Bothell Biotech Icos Keeps The Pipeline Full Of Promise”. The Seattle Times. p. F1. Retrieved January 10, 2009.
- Revill, Jo (February 2, 2003). “Drugs giant says its new pill will pack more punch than rival Viagra”. The Observer. Retrieved 2007-04-06.
- http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm274642.htm
- https://www.consumerreports.org/health/resources/pdf/best-buy-drugs/money-saving-guides/english/PillSplitting-FINAL.pdf
- “FDA Announces Revisions to Labels for Cialis, Levitra and Viagra”. Food and Drug Administration. 2007-10-18. Retrieved 2009-09-28.
- “Cialis: Warnings, Precautions, Pregnancy, Nursing, Abuse”. RxList. 2007. Retrieved 2007-04-06.
- Bischoff, E (June 2004). “Potency, selectivity, and consequences of nonselectivity of PDE inhibition”. International Journal of Impotence Research 16: S11–4.doi:10.1038/sj.ijir.3901208 . PMID 15224129. Retrieved January 19, 2009.
- Elliott, Stuart (January 10, 2006). “For Impotence Drugs, Less Wink-Wink”. The New York Times. p. C2. Retrieved January 15, 2009.
- Elliott, Stuart (April 25, 2004). “Viagra and the Battle of the Awkward Ads”. The New York Times. p. 1. Retrieved January 15, 2009.
- McCarthy, Shawn (March 5, 2005). “First they tried to play it safe; Ads for erectile dysfunction drug Cialis bared all – including a scary potential side effect. It was risky but it has paid off”. The Globe and Mail. p. B4.
- Loyd, Linda (July 6, 2003). “Two Pills Look to Topple Viagra’s Reign in Market; Levitra Expects Approval Next Month, Cialis Later This Year”. The Philadelphia Inquirer. p. E01.
- 38 is 1 below
- 39 is 2 below
- 40 is 3 below
-
- daugan, A. C.-M. Tetracyclic Derivatives; Process of Preparation and Use. U.S. Patent 5,859,006, Jan 12, 1999.
- Daugan, A. C.-M. Tetracyclic Derivatives, Process of Preparation and Use. U.S. Patent 6,025,494, Feb 15, 2000.
- Shi, X.-X.; Liu, S.-L.; Xu, W.; Xu, Y.-L. Tetrahedron: Asymmetry 2008, 19, 435–442.doi:10.1016/j.tetasy.2007.12.017
DAUGAN A ET AL: “THE DISCOVERY OF TADALAFIL: A NOVEL AND HIGHLY SELECTIVE PDE5 INHIBITOR. 2: 2,3,6,7,12,12A-HEXAHYDROPYRAZINO[1′,2′:1,6 ÜPYRIDO[3,4-B ÜINDOLE-1,4-DIONE” JOURNAL OF MEDICINAL CHEMISTRY, AMERICAN CHEMICAL SOCIETY. WASHINGTON, US, vol. 46, no. 21, 2003, pages 4533-4542, XP008052656 ISSN: 0022-2623
- Tadalafil bound to proteins in the PDB
- National Institutes of Health – Medlineplus
- Material Safety Data Sheet PDF file
- Official Cialis (Tadalafil) Website
- U.S. National Library of Medicine: Drug Information Portal – Tadalafil
13 C NMR OF TADALAFIL
COSY NMR OF TADALAFIL
DEPT NMR OF TADALAFIL
HSQC NMR OF TADALAFIL
HMBC NMR OF TADALAFIL
MASS SPECTRUM OF TADALAFIL
UV OF TADALAFIL
RAMAN SPEC OF TADALAFIL
| WO2009004557A2 * | Jun 28, 2008 | Jan 8, 2009 | Ranbaxy Lab Ltd | A process for the preparation of intermediates of tetracyclic compounds |
| WO2009148341A1 | Jun 3, 2009 | Dec 10, 2009 | Zaklady Farmaceutyczne Polpharma Sa | Process for preparation of tadalafil |
| WO2012107549A1 | Feb 10, 2012 | Aug 16, 2012 | Interquim, S.A. | PROCESS FOR OBTAINING COMPOUNDS DERIVED FROM TETRAHYDRO-ß-CARBOLINE |
| EP2107059A1 | Mar 31, 2008 | Oct 7, 2009 | LEK Pharmaceuticals D.D. | Conversion of tryptophan into ß-carboline derivatives |
| US8445698 | Jun 28, 2008 | May 21, 2013 | Ranbaxy Laboratories Limited | Process for the preparation of an intermediate of tadalafil |





























![[1860-5397-7-57-i28]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i28.png?max-width=550&background=EEEEEE)



Sorry, the comment form is closed at this time.